Beta-Glucan as a Soluble Dietary Fiber Source The efficacy and function of yeast β - glucan
2025-01-13
Abstract
This paper examines the multifaceted nature of β-glucan, a significant dietary fiber (DF) with various applications. It begins with a detailed analysis of its complex polysaccharide structure, followed by a discussion of different sources such as oats, barley, mushrooms, and yeast, highlighting their unique compositions. The paper scrutinizes how β-glucan is absorbed and metabolized in the human body, emphasizing its potential health benefits.
Furthermore, it outlines the extraction and purification processes necessary to obtain high-quality β-glucan for use in food, pharmaceuticals, and cosmetics. The discussion also showcases β-glucan’s biofunctional roles in immune modulation, cholesterol regulation, and gastrointestinal health, supported by clinical studies.
Additionally, the review explores the dynamics of global trade by tracing the evolution of β-glucan from a niche ingredient to a widely recognized commodity. In summary, this paper provides a comprehensive scientific perspective on β-glucan, making it a valuable resource for researchers, professionals, and industries interested in its potential within the dietary fiber landscape.
Keywords: β-glucan, cereals, fungus, microbes, bioavailability, biofunctionalities, industrial applications
1. Introduction
Dietary fiber (DF) is widely recognized as an essential component of a healthy diet, offering numerous benefits for human health. The consumption of DF is crucial for preventing and managing various health-related issues. Since DFs are not digested or absorbed by the human digestive system, they serve as a substrate for the gut microbiota. DFs can be classified into soluble and insoluble types based on their physiological properties, such as solubility, viscosity, and fermentability.
Among the various types of DFs, beta (β)-glucan has emerged as a significant and versatile soluble fiber, known for its remarkable structural characteristics and wide range of biofunctional attributes. With the growing awareness of health benefits associated with dietary fiber, the incorporation of DFs, including β-glucans, into food products has gained considerable attention. These fibers are noted for being low-calorie, low-cholesterol, and low-fat.
β-glucans are polysaccharides made up of glucose units linked by β-glycosidic bonds. The unique structural properties of β-glucan, which include branching patterns, degree of polymerization, and molecular weight, can vary based on their source. These variations are important as they significantly influence the physicochemical properties and bioavailability of β-glucan, ultimately affecting its diverse biological effects. One of the major functions of β-glucan is its ability to form viscous solutions, which acts as a prebiotic, enhancing the digestion and absorption of various biomolecules, including micronutrients, in the small intestine, thereby providing various health benefits and functional applications.
β-glucan can be sourced from a variety of origins including cereals, fungi, bacteria, and seaweeds, with extensive research interest due to its significance in human nutrition and industrial applications. In cereal grains, β-glucans are primarily concentrated in the bran, aleurone, and sub-aleurone layers. Barley and oats are recognized as particularly rich sources of β-glucans compared to other grains like wheat, rice, buckwheat, millet, and amaranth.
The bioavailability of β-glucan—key to its physiological effects—depends on factors such as molecular weight, solubility, and the presence of other dietary components. Understanding these factors is crucial for optimizing the health-promoting properties of β-glucan. However, progress in bioavailability assays, both in vitro and in vivo, has been limited. Therefore, given its significance in the food industry, comprehensive research on the bioavailability of β-glucan is essential.
Moreover, advancements in technology have led to significant improvements in the extraction and purification processes of β-glucan from its natural sources, allowing for higher purity and yield. This review discusses these methodological advancements in detail, considering the best methods for both laboratory and industrial scales.
In addition to its role as a dietary fiber, β-glucan possesses various biofunctional attributes, including immunomodulatory, cholesterol-lowering, and prebiotic effects. This has generated considerable interest in exploring β-glucan’s potential in clinical research, particularly concerning conditions such as diabetes, cardiovascular diseases, and gastrointestinal disorders. The biochemical and molecular mechanisms behind its effects on these health conditions are discussed in detail.
In the industrial sector, β-glucan has found applications in a wide range of products, from functional foods and dietary supplements to pharmaceuticals and cosmetics. Its functional properties, such as thickening and gelling abilities, contribute to its versatile role in product formulation and development. Furthermore, the global trade of β-glucan has seen significant growth, fueled by increasing consumer awareness of its health benefits and its incorporation into various products. Understanding the dynamics of this global trade is crucial for industry stakeholders, policymakers, and researchers.
As societies around the world confront the rising challenges of diet-related health issues, interest in β-glucan as a source of soluble dietary fiber has intensified. This comprehensive review aims to provide an in-depth exploration of β-glucan, covering its structural characteristics, diverse sources, bioavailability in the human body, extraction and purification techniques, multifaceted biofunctional attributes, clinical research highlighting its health benefits, industrial applications, and its role in the global trade market. By synthesizing the latest scientific findings and industry trends, this review seeks to offer a holistic perspective on the multifaceted role of β-glucan in nutrition, health, and commerce, highlighting significant advances in understanding and leveraging the potential of this remarkable soluble dietary fiber.
2. Potential Sources of β-Glucan
The main sources of β-glucan, a valuable functional ingredient, include cereals such as oats, barley, sorghum, wheat, and rye. Among these, oats and barley contain a satisfactory and chemically similar polysaccharide known as (1→3), (1→4)-mixed linkage β-D-glucan. The β-glucan content can vary from one cultivar to another, influenced by specific environmental conditions. Generally, oats contain 6-8% β-glucan, while barley contains 4-10% (w/w).
Daily consumption of 3 grams of β-glucan has been shown to significantly reduce cholesterol levels in the blood. It also decreases the circulation of low-density lipoproteins (LDL), which are major risk factors for cardiovascular diseases. This effective daily dose can be obtained from 75 grams of whole grain oats (which have a minimum of 5.5% β-glucan) or 55 grams of oat bran (which contains 4% β-glucan).
In addition to cereals, certain mushroom varieties, such as oyster and shiitake mushrooms, are also good sources of β-glucan. The cell walls of these mushrooms are rich in long or short-chain polymers of glucose subunits with β-1,3 and β-1,6 linkages, which contribute to the linear and branching structures of β-glucan.
Notably, an extract from the edible oyster mushroom has been shown to reduce the release of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in vitro, when lipopolysaccharide (LPS)-challenged monocytes are treated with the extract. This effect has also been observed in vivo in mice challenged with LPS after receiving the mushroom extract.
Mushroom β-glucans remain undigested in the human gastrointestinal tract, reaching the bowel virtually unchanged. They form a gel at the mucosal surface, modulating biliary salt absorption and ultimately affecting gut microbiota. Additionally, fragments of β-glucans found in the serum are absorbed from the intestinal tract.
Table 1.
Different sources of β-glucan and their molecular characterization.
| Source | Common Name | M.wt (g/mol) | Linkage | Content | Solubility | Branching Frequency |
DP3:DP4 | References |
|---|---|---|---|---|---|---|---|---|
| Triticum aestivum | Wheat β-glucan | 4.3–75.8 × 104 | β-1,3 and β-1,4 | 0.18–1.8 (%w/w) |
Soluble | 2.8–4.5 | [9] | |
| Avena sativa | Oat β-glucan | 65–310 × 104 | β-1,3 and β-1,4 | 2.2–7.5 (%w/w) | Soluble | 1.5–2.3 | [10] | |
| Hordeum vulgare | Barley β-glucan | 31–270 × 104 | β-1,3 and β-1,4 | 2.2–19.8 (%w/w) |
Soluble | 1.8–3.4 | [11] | |
| Sorghum bicolor | Sorghum β-glucan | 36 × 104 | β-1,3 and β-1,4 | 0.1–1.7 (%w/w) | Soluble | 2.1–3 | [12] | |
| Secale cereale | Rye β-glucan | 21–110 × 104 | β-1,3 and β-1,4 | 1.0–2.7 (%w/w) | Soluble | 1.9–3 | [11] | |
| Grifola frondosa | Grifolan | 3 × 104 | β-1,3 and β-1,6 | Soluble | 1/3 | - | [13] | |
| Shizophyllan commune | Schizophyllan | 45 × 104 | β-1,3 and β-1,6 | Soluble | 1/3 | - | [14] | |
| Lentinus edodus | Lentinan | 30–40 × 104 | β-1,3 and β-1,6 | Soluble | 2/5 | - | [15] | |
| Saccharomyces cerevisiae | Zymosan | 24 × 104 | β-1,3 and β-1,6 | 5–7 (%w/v) | Insoluble | 0.03–0.2 | - | [16] |
| Euglena gracilis | Paramylon | 50 × 104 | β-1,3 | 90 (%w/v) | Insoluble | - | [17] | |
| Alcaligenes faecalis | Curdlan | 10 × 104 | β-1,3 | Unbranched | - | [18] |
Open in a new tab
3. Advances in the Biosynthetic Pathway of β-Glucan
Recent research has highlighted the dynamic nature of β-glucan levels in plants, which tend to increase during growth and developmental phases but decrease once growth ceases. In specific cereals like barley, oats, and rye, β-glucan is primarily found in the aleurone layer, where it plays a crucial role in germination. During this process, the enzyme β-glucanase is essential as it catalyzes the breakdown of β-glucan molecules, providing a vital source of carbohydrates for germinating seeds.
The biosynthesis of β-glucan involves a complex network of enzymes and genes closely related to the pathways for starch and cellulose. Notably, members of the cellulose synthase-like (CSL) subfamilies CSL A to H and J have been implicated in this biosynthesis. Central to the β-glucan biosynthesis pathway is the enzyme CSL, which catalyzes the formation of cellobiosyl units by linking two glucose residues. Following this, a glycosyl transferase enzyme transfers glycosyl residues onto cellobiosyl, forming cellotriosyl units and other odd-numbered units.
Extensive studies have underscored the crucial role of cellulose synthase-like F (CslF) genes in β-glucan biosynthesis. Gain-of-function experiments using transgenic approaches and loss-of-function experiments with RNA interference (RNAi) have demonstrated their significance. Among these genes, CslF6 stands out as the primary gene responsible for β-glucan synthesis. For example, one study reported the direct involvement of HvCslF6, HvCslH1, and HvCslJ in β-glucan biosynthesis by generating mutants with reduced expression of CslF6. Furthermore, research in barley using CRISPR/Cas9 to induce mutations in the CslF6 and CslH1 genes revealed their critical roles in cell wall polysaccharide synthesis. Frame shift mutations in these genes led to changes in polysaccharide content and grain characteristics, further emphasizing the importance of HvCslF6.
Table 2.
Genes involved in the synthesis of β-Glucan in cereals.
| Gene | Gene Source | Promoter | Host | Outcome | Refernces |
|---|---|---|---|---|---|
| HvCslF4 | Hordeum vulgare | CaMV 35S | Hordeum vulgare | Increase in DP3/DP4 ratio and β-glucan in grain (upto 50%) | [24] |
| HvCslF6 | Hordeum vulgare | CaMV 35S | Nicotiana benthamiana | Loss of the β-1,3 and β-1,4 linkage activity, hence lack of β-glucan in leaves | [25] |
| HvCslF6 and HvCslH1 HvCslF3 and HvCslF9 |
Hordeum vulgare | OsU6snRNA | Hordeum vulgare | Lesser β-glucan, decrease in DP3:DP4 ratio, grain test weight decreased | [23] |
| BdCSLF6 | Brachypodium distachyon | CaMV 35S | Nicotiana benthamiana | Alteration in carbon metabolism and reduction in grain β-glucan content | [26] |
| OsCslF6 | Oryza Sativa | Cell wall specific promoter, CaMV 35S | Arabidopsis | Overexpression leads to accumulation of β-glucan and reduced growth | [27] |
| OsCslF2, OsCslF4 | Oryza Sativa | CaMV 35S | Arabidopsis | Synthesize β-glucan in leaves | [28] |
| TaCslF6 | Triticum aestivum | Endosperm specific | Triticum aestivum | 30% reduction inβ-glucan in endosperm | [22] |
| TaCslF6 | Triticum aestivum | CaMV 35S | Nicotiana benthamiana | Synthesize β-glucan in leaves | [29] |
Open in a new tab
Figure 1.
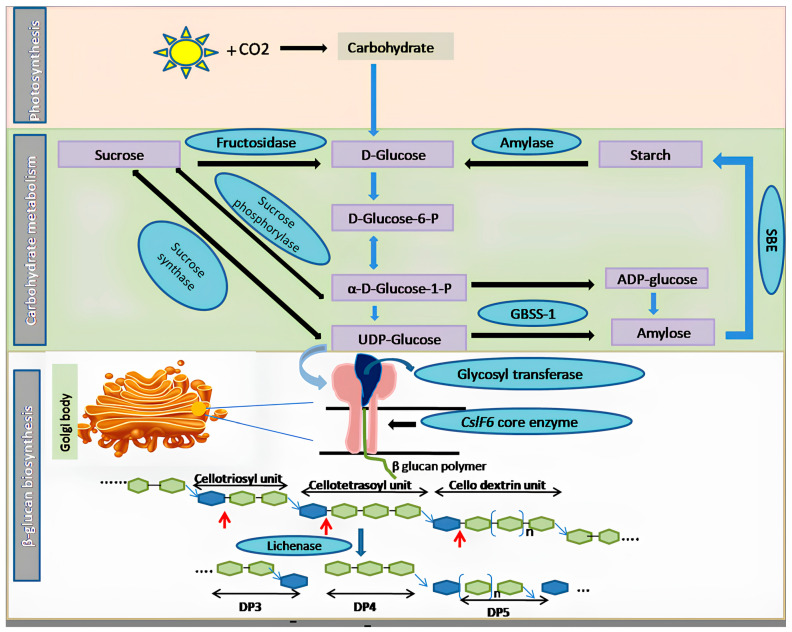
**Schematic Presentation of the β-Glucan Biosynthetic Pathway in Cereals**
The β-glucan biosynthetic pathway in cereals involves various components, including carbon dioxide (CO2), D-Glucose-6-Phosphate (D-Glucose-6-P), α-D-Glucose-1-Phosphate (α-D-Glucose-1-P), uridine diphosphate (UDP-Glucose), starch branching enzyme (SBE), granule-bound starch synthase-1 (GBSS-1), cellulose synthase-like family 6 (CslF6), and degree of polymerization (DP). Red arrows indicate the action sites of the lichenase enzyme.
A significant advancement was made when reference [28] introduced the CslF2 and CslF4 genes from rice into Arabidopsis, which typically lacks β-glucan in its cell wall. This genetic manipulation resulted in the successful synthesis of β-glucan within the Arabidopsis cell walls, confirmed through enzymatic assays and specific antibodies. Additionally, reference [27] confirmed the role of CslF6 in the biosynthesis of mixed linkage β-glucan in rice. Interestingly, loss-of-function mutations in these β-glucan synthesis genes were found to increase disease susceptibility, highlighting the importance of these genes in enhancing the immune and defense systems [28].
Research by reference [30] provided valuable insights into the biochemical characteristics and spatial organization of β-glucan in cereals and grasses. Their findings revealed distinct synthesis patterns for various cell wall polysaccharides, showing that cellulose is synthesized at the plasma membrane, while mixed polysaccharides are produced within Golgi bodies. Furthermore, CslF6 was identified as the key enzyme responsible for generating cello-dextrins, which are subsequently linked at the plasma membrane to form β-D-glucan chains.
Further investigations by reference [31] identified seven genomic regions in tetraploid wheat associated with β-D-glucan content. Marker trait association (MTA) analyses across various grass species implicated potential candidate genes in regulating β-glucan synthesis, particularly concerning carbon resource allocation. Additionally, reference [20] expanded the understanding of β-glucan biosynthesis by identifying 14 MTA and seven genes encoding enzymes involved in glucose metabolism linked to β-glucan production in barley. Transcriptome studies revealed differentially expressed genes associated with hydrolase activity, starch synthesis, and β-glucan metabolism, all intricately linked to β-glucan biosynthesis [21].
In vitro experiments have clearly demonstrated that UDP-glucose, in the presence of Mg2+, serves as a precursor for β-glucan synthesis, catalyzed by the CslF6 enzyme. The lichenase enzyme was used to hydrolyze the 3H-labeled polymer to confirm the linkage between glucosyl subunits within the synthesized polymer. This enzymatic digestion resulted in the production of DP3 and DP4 fragments, indicating specific cleavage of β-1,4 linkages followed by β-1,3 linkages, providing strong evidence for the role of the CslF6 gene in β-glucan synthesis.
Beyond the plant kingdom, β-glucan is primarily found in the cell wall and division septum of yeast, where it plays a crucial role in providing structural rigidity and fortifying the cell wall. Immunoelectron microscopy has revealed the accumulation of β-(1,6)-glucan particles associated with the Golgi apparatus in the yeast species Schizosaccharomyces pombe. This suggests that β-(1,3)-glucan binds to the cell surface, while the initial production of β-(1,6)-glucan occurs in the Golgi. In contrast to cereals, where CslF6 is responsible for β-(1,3)-glucan synthesis, yeast employs an enzyme complex known as β-(1,3)-glucan synthase (BGS), located on the cytosolic face of the plasma membrane. BGS catalyzes the production of β-(1,3)-glucan using UDP-glucose as a substrate (Figure 2).
In vitro experiments have demonstrated that this enzyme generates linear chains composed of approximately 80 glucose molecules, which are then elongated and branched in yeast species like Saccharomyces cerevisiae with the help of glycosylphosphatidylinositol (GPI)-bound Gas proteins. These proteins belong to the glycosidase/transglycosidase 72 family. Several other genes involved in the biosynthesis of β-glucan in yeast are outlined in Supplementary Table S1. An in vitro assay was employed to identify the subunits of BGS, where acid-insoluble radioactive β-(1,3)-glucan accumulates when membrane extracts are treated with radiolabeled UDP-glucose and GTP. A similar experiment separated BGS into membrane-bound and cytosolic components, which each carry the catalytic and regulatory GTP-binding modules [32].
Fission yeast possesses four genes, named bgs1 to bgs4, encoding four putative BGS catalytic subunits. Among these, bgs1, bgs3, and bgs4 are essential for vegetative growth, while bgs2 is involved in spore wall formation and sexual differentiation. A critical regulatory component in this process is the GTPase Rho1, a Ras-like GTP-binding protein that activates the BGS complex by binding to GTP. Collectively, findings from various organisms strongly support the role of BGS proteins in catalyzing β-(1,3)-D-glucan chain biosynthesis in vivo. However, it is worth noting that...
Figure 2.
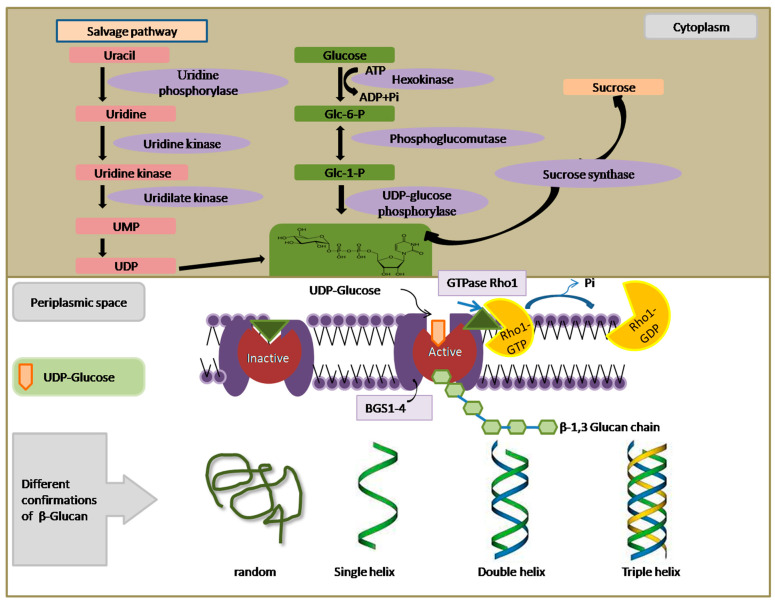
Open in a new tab
This model illustrates the β-glucan biosynthetic pathway in yeast. Key components include UMP (uridine monophosphate), UDP (uridine diphosphate), Glc-6-P (glucose-6-phosphate), Glc-1-P (glucose-1-phosphate), BGS (β-glucan synthase), ATP (adenosine triphosphate), ADP (adenosine diphosphate), GTP (guanosine triphosphate), GDP (guanosine diphosphate), Pi (inorganic phosphate), and Rho 1 (a Ras-like GTP-binding protein).
In essence, the biosynthesis of β-glucan is a complex and finely orchestrated process that plays significant roles in plant growth, development, and defense mechanisms. The intricate interplay of enzymes and genes involved in the synthesis and modulation of β-glucan is a key focus of research within the field of plant biology. These insights shed light on fundamental processes in plants and hold promise for potential applications in agriculture and nutrition.
Future research in β-glucan biosynthesis is set to explore several exciting avenues. There is an increasing demand to understand the intricate regulatory mechanisms that govern β-glucan production in cereals, paying particular attention to genetic and epigenetic regulation. Additionally, advancements in genome editing technologies, such as CRISPR/Cas9, provide opportunities for the precise engineering of β-glucan content and structure in crops, which could enhance their nutritional value and effectiveness in industrial applications. Furthermore, investigating the impact of manipulating β-glucan on plant growth, development, and immunity will deepen our knowledge of plant biology.
In yeast, supporting evidence from purified active membrane-bound subunits will be essential to definitively demonstrate that the Bgs/Fks proteins are the catalytic subunits of BGS. This research will provide concrete proof of how each Bgs protein uniquely biosynthesizes its respective β-D-glucan.
4. Methodological Advances in Extraction and Purification of β-Glucan from Different Sources
4.1. Advances in Extraction Procedure
The achievement of high-purity β-glucan is a critical concern for both laboratory and commercial applications. This focus is particularly on factors such as purity, molecular integrity, and yield. The extraction method used plays a significant role in determining the molecular weight and structural characteristics of β-glucan. Discrepancies in the estimated molecular weight of β-glucan can result from variations in extraction and purification procedures, aggregation phenomena, and depolymerization events that occur during the extraction process. Consequently, extraction methods should aim to maintain the integrity of the β-glucan molecules while maximizing both yield and purity to effectively assess the structural properties of the isolated β-glucans. Since β-glucan's discovery, numerous extraction techniques have been developed and optimized to enhance yield. Common wet extraction methods include aqueous, alkaline, acidic, and enzymatic techniques. Among these, hot water extraction is the most widely utilized. However, wet extraction methods have their drawbacks, including lengthy extraction times, high operational costs, and environmental concerns. These methods use water, acidified water, or alkali to solubilize β-glucan by hydrolyzing whole grains such as barley or oats. After solubilization, centrifugation and ethanol precipitation are employed to separate the β-glucan from the slurry. The use of ethanol may inhibit native enzymes, which can lead to the removal of nonpolar molecules and the dissociation of proteins and sugars. Following the initial rough extraction, further refinement is performed to eliminate contaminants like starch, protein, and fat. Various extraction and purification techniques for β-glucan are illustrated in Figure 3. Alternatively, dry separation techniques such as pearling and air classification are commonly employed to avoid using solvents. However, these dry techniques typically result in lower β-glucan recovery rates (less than 30%) compared to wet methods, which can achieve a recovery range of 20 to 70%.
Figure 3.
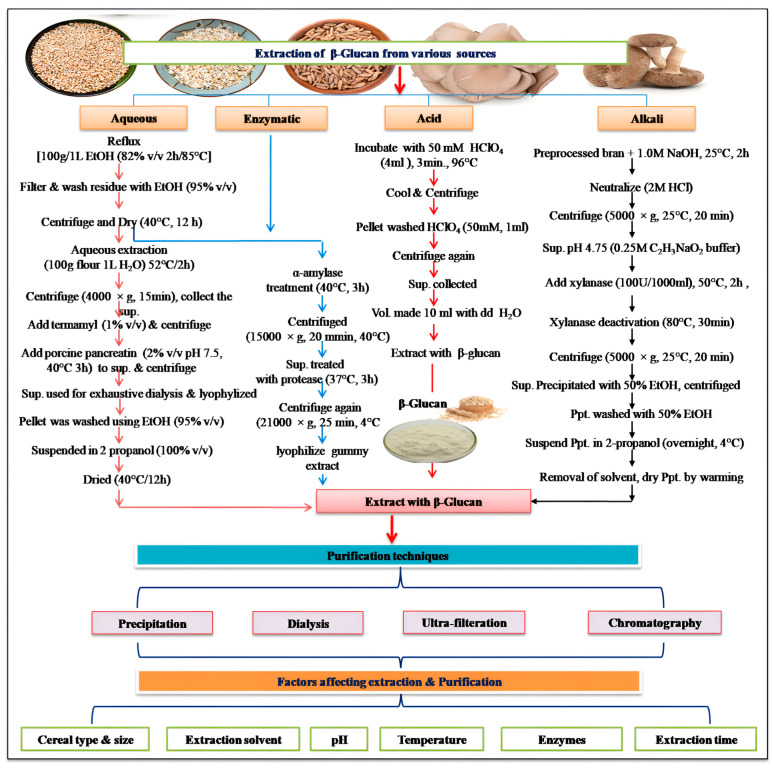
Open in a new tab
Extraction and purification of β-Glucan, along with the factors influencing their extraction and purification. Abbreviations: EtOH: ethanol, HClO4: perchloric acid, Vol.: volume, NaOH: sodium hydroxide, HCl: hydrochloric acid, C2H3NaO2: sodium acetate, dd H2O: double distilled water, Ppt.: precipitates, Sup.: supernatant.
A commercially viable and environmentally friendly extraction process for β-glucan would address the challenges currently faced in its extraction. Recently, two innovative techniques—superheated water extraction and rapid solvent extraction—have been developed. These methods are not only commercially viable but also environmentally friendly, and they offer improved yields of β-glucan.
Choosing the appropriate extraction technique depends on several factors, including the source of raw materials, the desired purity levels, and cost considerations (see Supplementary Table S2). Different cereals have varying β-glucan content and extraction efficiency, with barley and oats being the top sources, followed by wheat, rye, and corn.
Particle size is a crucial factor, as smaller particle dimensions increase surface area and enhance extraction efficiency. The choice of extraction solvent is also important; water, ethanol, and acetone are commonly used, depending on the type of β-glucan and the purity goals.
Moreover, adjusting the pH of the extraction solution has a significant effect on β-glucan solubility, with alkaline solutions proving more effective than acidic ones. Temperature is another critical factor, as it affects the extraction rate. However, it must be carefully controlled to avoid degrading β-glucan.
The enzymatic content in the extraction solution should be inactivated using heat treatment or enzyme inhibitors. Extraction time influences β-glucan yield significantly—longer extraction times generally result in higher yields, although they also increase the risk of damaging the β-glucan.
It is important to note that solubilized β-glucan is vulnerable to molecular fragmentation caused by shear forces during mixing and centrifugation, as well as enzymatic degradation from intrinsic β-glucanases present in the solution. This enzymatic breakdown and shear-induced fragmentation can lead to reduced molecular weight and viscosity of the β-glucan, compromising its potential cholesterol-lowering and blood sugar-lowering effects.
In the following sections, we will discuss some commonly used techniques for extracting β-glucan in detail.
4.1.1. Aqueous Extraction
Aqueous extraction is a widely used method for isolating β-glucan from cereals due to its simplicity and cost-effectiveness. This process involves grinding cereals into a fine powder, which is then mixed with water. The mixture is heated to a temperature typically ranging from 50 to 60 °C. After heating, insoluble components are removed through filtration, and the β-glucan is concentrated by evaporation.
The temperature during the aqueous extraction process is crucial for β-glucan recovery. Reference [36] noted a positive correlation between temperature and β-glucan yield, achieving a maximum extraction efficiency of up to 89.1%. Therefore, the use of thermally stable α-amylase to hydrolyze starch during hot water extraction, as suggested by Reference [36], is significant. For barley, the extraction of β-glucan uses water as the solvent.
Alternatively, Reference [37] investigated a method for extracting β-glucan from oats and barley. This approach involves grinding the cereal grains into flour, which is then mixed with water to produce a slurry containing an aqueous β-glucan solution along with solid residue. The aqueous solution is separated from the solid residue, and water is removed through evaporation, ultrafiltration, or a combination of methods to obtain a gel or solid enriched with β-glucan.
Despite the advantages of aqueous extraction—such as speed, simplicity, and affordability—there are limitations to consider. This method is generally less efficient and may compromise the biological activity of β-glucan compared to alternative techniques. Therefore, further research is needed to optimize extraction procedures to achieve both maximum recovery and enhanced biological activity.
4.1.2. Alkaline, Acidic and Enzymatic Extraction
Over the years, the extraction of β-glucan has seen several advancements, with alkaline extraction becoming more prominent than aqueous extraction due to its greater effectiveness. However, this method has a potential drawback: it can compromise the structural integrity and biological activity of β-glucan. Additionally, alkaline extraction tends to be more expensive than aqueous extraction and requires the use of inorganic chemicals such as potassium or sodium hydroxide. The process involves prolonged stirring, followed by filtration to remove insoluble components, and evaporation to concentrate the β-glucan. In contrast, acidic extraction is a gentler but less effective method, posing a lower risk of damaging β-glucan during extraction. This method involves mixing finely ground cereal raw materials with an acidic solution, typically containing sulfuric or hydrochloric acid. The mixture is heated to a temperature range of 50 to 60 °C while being continuously stirred, followed by filtration to eliminate insoluble fractions. Acidic extraction is more cost-effective than enzymatic extraction and has a lower risk of damaging β-glucan. Among the various extraction techniques, enzymatic extraction is considered the most effective, yet it is also the most expensive. This method involves mixing grain powder with an enzyme that selectively breaks down β-glucan without significantly altering its original structure. However, it is mainly suitable for small-scale experimental applications. Recently, alternative methods such as accelerated solvent extraction (ASE), reflux extraction, microwave-assisted extraction (MAE), and ultrasound-assisted extraction (UAE) have emerged as effective means for extracting high-quality β-glucan from various cereal sources. ASE, in particular, offers an environmentally friendly and efficient approach, yielding higher quantities of β-glucan in a shorter extraction time. Overall, these advancements aim to improve the extraction and preservation of β-glucan from diverse cereal sources. Despite this progress, the search for a robust, cost-effective, reproducible, and eco-friendly industrial-scale extraction method remains a significant challenge for the future.
4.2. Advances in Purification Procedure
Recent advancements in β-glucan purification have significantly elevated the field, leading to innovations that have transformed its separation and refinement processes. These breakthroughs have increased the purity of β-glucan, making it suitable for a broader range of applications. Researchers have utilized cutting-edge techniques to enhance the efficiency and precision of β-glucan purification, effectively addressing challenges related to impurities and yield. One common method for purifying β-glucan involves using a suitable solvent to induce precipitation, with ethanol being the most widely used due to its compatibility with water. The precipitation process occurs in two sequential steps: first, β-glucan is dissolved in the solvent, and then β-glucan molecules aggregate and precipitate due to the solvent's nonpolar nature. To isolate the precipitate from the solution, centrifugation or filtration is employed. The choice of solvent is influenced by the solubility of β-glucan; when water fails, ethanol or acetone can be used as alternatives. Despite this, water generally remains the ideal solvent for β-glucan precipitation. The concentration of β-glucan in the solution directly affects the effectiveness of the precipitation process, as higher concentrations increase the likelihood of successful precipitation. This method is notably fast and cost-effective, efficiently removing various impurities from β-glucan solutions, although time constraints and the potential risk of β-glucan degradation may limit its application. Dialysis is another purification technique that separates molecules based on size. In β-glucan purification, dialysis involves passing the solution through a semipermeable membrane that allows small molecules and water to pass while blocking β-glucan. Cellulose acetate membranes are commonly used for this purpose, as they are impermeable to β-glucan while allowing the passage of water and small molecules. Dialysis is a gentle method for removing contaminants from the β-glucan solution, often followed by ethanol and ammonium sulfate precipitation. Additionally, dialysis can extract glucose, peptides, and amino acids from β-glucan, thus increasing the final product's purity. While dialysis is time-consuming and may not be ideal for large-scale production, it produces a product with superior purity and viscosity compared to alternative methods. Nonetheless, this approach requires additional time and specialized equipment. Ultrafiltration has emerged as a time-saving purification method that uses semipermeable membranes with pore sizes ranging from 250 to 500 nm. This method employs strong shear forces, resulting in reduced viscosity and molecular weight of β-glucan. Among the various techniques explored, a combination of alcohol extraction and ammonium sulfate precipitation has been particularly effective, achieving a remarkable 91.38% purity, despite a moderate yield. Chromatography has also become a well-regarded method for β-glucan purification, providing meticulous separation of its constituents. Gel filtration chromatography is a popular choice, involving columns filled with porous gel matrices of varying sizes. β-glucan molecules adhere to these matrices based on their size, allowing for the sequential elution of smaller and larger molecules. Although this technique is highly effective at enhancing β-glucan purity, it poses challenges in terms of time and cost. Furthermore, chromatography is a more complex approach that requires suspending the β-glucan solution in a solid matrix column; the specific binding of solution components to the matrix facilitates separation through solvent elution. Various chromatographic methods, such as gel filtration, ion exchange, and affinity chromatography, can be tailored to the unique characteristics of β-glucan and the desired purity levels. In summary, recent innovations in β-glucan purification have greatly improved its purity and versatility across various industries. Techniques such as precipitation, dialysis, ultrafiltration, and chromatography effectively address challenges related to impurities and yield. While precipitation is speedily cost-effective, dialysis offers superior purity, ultrafiltration reduces viscosity, and chromatography ensures precise separation. However, all these methods require time and resources, which must be taken into consideration.
5. Deciphering Structure-Function Characteristics and Types of β-Glucan
β-glucan has gained recognition as a bioactive food ingredient due to its various biological activities. It is found in a wide range of sources, including bacteria, algae, barley, yeast, mushrooms, and oats. The structural details of β-glucan molecules, as shown in Figure 4, demonstrate significant variations depending on their source. These polymers are composed of glucose subunits linked by β-(1,3) or β-(1,4) glycosidic bonds, often featuring branches at the six-position of the backbone. For example, β-glucans derived from mushrooms typically have short β-(1,6)-linked branches, whereas those from yeast have β-(1,6) side branches along with additional β-(1,3) regions. β-glucans exhibit a remarkable combination of properties, merging the functional characteristics of viscous and gel-forming food hydrocolloids with the physiological benefits of dietary fibers. Research into the properties of β-glucan began in the 1950s, focusing initially on dried yeast β-glucan extracted from Saccharomyces cerevisiae. The characterization of its structure involved detailed analyses of total sugar content and molecular weight, using advanced techniques such as multi-angle laser light scattering alongside size exclusion chromatography.
Figure 4.
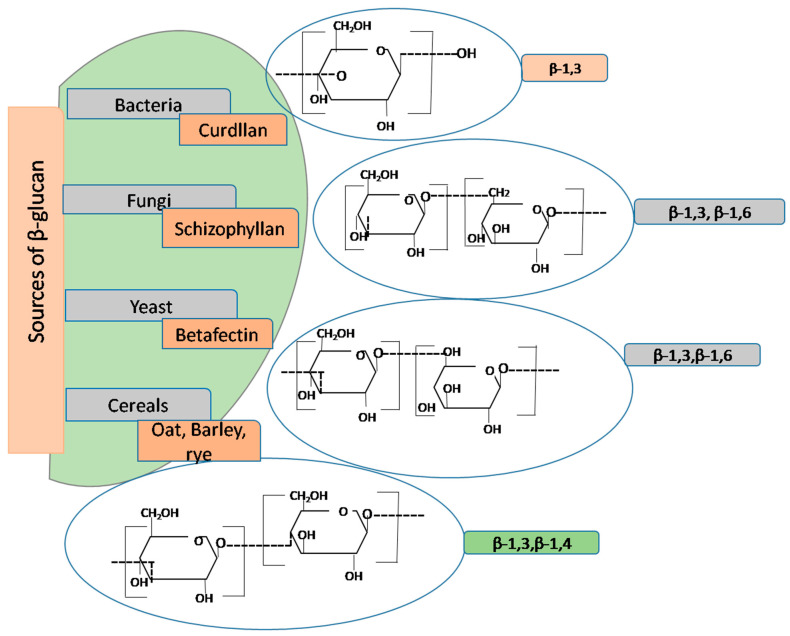
Open in a new tab
**Schematic Model of β-Glucan Structure and Branching from Various Sources**
Fungal species are notably characterized by the significant presence of chitin and β-glucan in their cell walls and intracellular structures. The arrangement of cellodextrin, a critical component, is intricately linked to the concentration of the substrate UDP-glucose pyrophosphate (UDP-GluP). When UDP-GluP concentrations are low, the activity of glycosyl transferases is hindered, which impedes the transfer of glucose residues to the cellobiose unit [30].
The molecular weight of β-glucan, along with its water retention properties and solubility, profoundly influences its viscosity. Solubility is dependent on the molecular weight of β-glucan, which can vary broadly based on the source and extraction method. Research has shown that the molecular weight of β-glucans from oats and barley ranges from 130 kDa to 390 kDa and from 190 kDa to 410 kDa, respectively. Moreover, some β-glucans exhibit structural variations, characterized by distinct branching patterns, linkages, or the attachment of other molecules, such as proteins, as observed in polysaccharide-K (PSK, Krestin) and polysaccharide-peptide. β-Glucan, composed of β-D-glucose monomer units linked by glycosidic bonds at positions β(1→3), (1→4), and/or (1→6), can exist in both branched and unbranched configurations, and it shows great promise in promoting health. Its beneficial effects include lowering the glycemic index, managing cholesterol levels, and reducing the risk of various cardiovascular diseases.
The molecular weight, shape, and structure of β-glucans—affected by extraction techniques—play a crucial role in initiating diverse biological activities, particularly immunological responses. In aqueous solutions, β-glucans can assume triplex helix, singlet, or duplex conformations, with the immune potency believed to depend on both conformational complexity and molecular weight. To fully exploit the various activities and attributes of β-glucan, an increasing number of research initiatives are investigating innovative strategies to modify its conformation. These approaches include physical methods that involve temperature variations, chemical methods using acids, alkalis, and salts, enzymatic methods utilizing enzymes like glucanase and lichenase, and mechanical techniques such as homogenization and sonication. These research avenues show promise for optimizing the potential applications of β-glucan across different fields.
When subjected to enzymatic digestion with lichenase, β-glucans produce oligomeric products, including trisaccharide units (degree of polymerization; DP-3) known as cellobiosyl-D-glucose, tetrasaccharide units (DP-4) called cellotriosyl, and longer cellulose-like cellodextrin units (DP > 5). These cellodextrin units have a tendency to aggregate, precipitating out of the β-glucan chain, thus complicating quantitative analysis. The oligosaccharides may exist in either a random or non-random arrangement within the β-glucan chain. Interestingly, cereals, despite their similar molecular structure, display variations in the ratios of DP3 to DP4 and the ratio of cellotriosyl to cellotetrosyl, as well as their molecular size. For instance, wheat has a higher DP3/DP4 ratio (3–4.5), while barley shows a lower ratio (2.4–2.5), and oats fall somewhere in between at a ratio of 1.6 to 2.3 [43].
Environmental factors, including growth conditions, can influence the degree of polymerization and the DP3:DP4 ratio. Additionally, the molecular weight of β-glucan can be affected by environmental elements, extraction and purification methods, and genetic factors. This variation in molecular weight is a critical determinant of β-glucan’s physical properties, such as viscosity and solubility, which in turn play a central role in shaping its functionalities. Understanding these intricate structural nuances is essential for appreciating the diverse health implications of β-glucan and optimizing its use in various applications.
Lentinan, a β-glucan derived from the fungus Lentinus edodes, exhibits a remarkable structural feature: it forms triple helical structures at room temperature. This unique characteristic gives it high viscosity and an exceptional ability to withstand a wide range of environmental conditions, including variations in pH, temperature, and salt concentration when dissolved in aqueous solutions. This property makes lentinan particularly interesting for both researchers and industry professionals. [44] highlights the intriguing conformational diversity within β-glucans, noting that high molecular weight β-glucans, like schizophyllan, can adopt both single and triple helical conformations. In contrast, low molecular weight β-glucans tend to exhibit a random coiled conformation. These molecular and structural characteristics are vital, as they significantly impact the physical properties of β-glucans, including their solubility and rheological behavior, as well as their functional effects when incorporated into food products. Low molecular weight yeast β-glucans, in particular, demonstrate superior antioxidant and immunological properties.
6. Advances in Bioavailability Studies of β-Glucan
Bioavailability is a crucial parameter that measures the extent to which a consumed substance reaches its intended target, exerts its effects, and gets utilized. This metric is particularly important in the fields of pharmacology and nutritional science. Research on the bioavailability of soluble β-glucans has been relatively limited, making it a focal point for further investigation.
A study investigating the oral administration and gastrointestinal absorption of soluble β-glucans found that their bioavailability ranges from 0.5% to 4.9%. Among the three types of soluble β-glucans studied, laminarin had the highest bioavailability, followed by scleroglucan, while glucan phosphate exhibited the lowest bioavailability at 0.5%. Another examination focused on the metabolism of barley β-glucans in the upper intestine, using ileal effluents collected from a diet containing barley. The results were compared to in vitro β-glucan extraction procedures. In vitro studies showed that endogenous proteases solubilized approximately 28% of the β-glucan, which increased to 83% after separating non-starch polysaccharides (NSP). Similarly, around 60% of the β-glucan was solubilized in the ileal effluent. Notably, the viscosity of the ileal effluent remained low, comparable to a mucin benchmark. These findings suggest that although β-glucan can be solubilized in the upper intestine, its viscosity might not significantly affect its bioavailability.
A separate study examined the metabolism and bioavailability of hydrogenated resistant glucan (HRG) and resistant glucan (RG) in both rats and humans. Unlike many high molecular weight carbohydrates that are partially digested and mildly fermented in healthy individuals, RG and HRG showed limited digestion and fermentation. As a result, both RG and HRG exhibited very low bioavailability in both human and rat subjects. This reduced bioavailability is attributed to the high mobility of RG among intestinal bacteria, while the oligosaccharides in pure RG had limited mobility of their primary components.
Another investigation explored the impact of acetylation—with acetic anhydride at concentrations of 4–6%—on oat β-glucan. This study assessed changes in the thermal, morphological, functional, and rheological properties of β-glucan concentrates containing 31% oat β-glucan. Acetylation of the β-glucan molecule reduced its capacity to bind fat and enhanced glucose availability. Notably, the increased ability to bind bile acids and greater swelling power contributed to these functional improvements.
Recent research using rat models indicates that the bioavailability of β-glucans falls within the 4–5% range, with soluble glucans capable of moving from the gastrointestinal tract into systemic circulation. Various intestinal cell types, including mucosal dendritic and epithelial cells, are known to interact with β-glucans, although the precise mechanisms underlying these interactions are still not fully understood.
Current research highlights that β-glucan metabolism and bioavailability can vary based on their source and structure. Factors such as enzymatic solubilization in the gastrointestinal tract and interactions with gut bacteria significantly influence bioavailability. Additionally, acetylation is being explored as a method to modify β-glucans, which may affect their functionality and bioavailability.
Future research areas include investigating the mechanisms of absorption and metabolism, exploring differences based on source and structure, continuing studies on functional modifications, and examining health implications. Understanding β-glucan bioavailability is crucial for optimizing its therapeutic potential, and ongoing research aims to address existing knowledge gaps and enhance the application of β-glucans in various health-related contexts.
7. Mechanistic Insights on Biofunctionalities of β-Glucan
7.1. Mechanism of Hypoglycemic Effect
Diabetes is a chronic condition characterized by elevated blood sugar levels and is known to be a precursor to various cardiovascular diseases, including hypertension, coronary artery disease, peripheral vascular disease, and atherosclerosis. The World Health Organization (WHO) reports a concerning increase in the global prevalence of type II diabetes. The causes of diabetes are multifaceted, involving factors such as dysfunctional beta (β) cells, insulin resistance, abnormal mitochondrial activity, and cell death (apoptosis).
β-glucan has emerged as a crucial agent in improving insulin activity and lowering postprandial blood glucose levels. Researchers have been investigating the health benefits of β-glucans derived primarily from cereals like barley and oats. These compounds have gained attention for their ability to lower blood sugar and cholesterol levels while modulating insulin responses. Barley, in particular, is notable for its low glycemic index compared to other cereals, making it a valuable dietary choice. For instance, adding just 4 grams of barley-derived β-glucan to chapattis reduced their glycemic index from 54 to 30. The mechanism behind this effect involves β-glucan acting as dietary fiber (DF), which delays gastric emptying and slows the release of glucose, ultimately leading to lower blood glucose levels. Oat-derived β-glucan has similarly demonstrated the capacity to lower blood sugar and insulin levels. It has also been observed that oat β-glucan can hinder enzyme activity, resulting in decreased starch digestion and lower postprandial glucose levels. Its gel-forming properties in the small intestine delay the interaction of digestive enzymes with nutrients, reducing the absorption of glucose and temporarily lowering peak postprandial blood glucose concentrations.
In addition to its glycemic control properties, β-glucan has been linked to the reduction of menaquinol, a form of vitamin K2 associated with type 2 diabetes. Studies have shown that incorporating natural oat products rich in β-glucans into the diets of individuals with diabetes can improve glucose levels, especially when consumed in higher daily doses.
As a dietary fiber, β-glucan avoids digestion in the small intestine and is fermented in the large intestine, producing short-chain fatty acids (SCFAs). These SCFAs help maintain gut pH and regulate the secretion of gut hormones, playing a significant role in signaling satiety. Research has demonstrated the anti-diabetic potential of β-glucans, showing reductions in cholesterol, triglycerides, and glucose levels in obese rats. Oat β-glucans have been found to decrease the activity of key intestinal disaccharidases, thereby lowering glycemic responses in a dose-dependent manner in both in vivo and in vitro studies, especially when these sugars are mixed with high-viscosity β-glucan gel. The viscosity of this gel is inversely correlated with spikes in post-meal blood glucose. Studies involving mice suggest that the effects of endogenous β-glucanases on hyperglycemia and LDL cholesterol management remain unaffected by the incomplete hydrolysis of barley β-glucans. Additionally, the expression of the enzyme α-glycosidase is inhibited, and the expression of glucose transporters SGLT1 and GLUT2 is reduced. These factors help regulate carbohydrate absorption and elevate GLP-1 levels.
A meta-analysis encompassing 17 trials with 68 distinct protocols and 212 individuals found that the consumption of barley β-glucan significantly reduced postprandial glycemic reactions, indicating a scientifically meaningful reduction in blood sugar levels.
Overall, β-glucans from cereals like barley and oats show great promise in managing blood sugar and cholesterol levels and improving insulin responses through various mechanisms. This makes them a topic of considerable scientific interest and potential clinical relevance. Future research could explore personalized β-glucan interventions, clarify specific molecular pathways, and develop innovative β-glucan-enriched food products to optimize glycemic control, cholesterol management, and metabolic health for individuals with diverse dietary and genetic profiles.
7.2. Mechanism of the Cholesterol Lowering Effect
Dyslipidemia, a chronic condition characterized by elevated lipid and cholesterol levels in the bloodstream, poses a significant risk for the development of life-threatening diseases such as myocardial infarction, ischemic and hemorrhagic stroke, and atherosclerotic vascular disease. It is imperative to investigate potential treatments to mitigate the rising mortality associated with dyslipidemia. The American Heart Association reported that 11.7% of individuals aged 20 or older, totaling 28.5 million people, have blood total cholesterol levels exceeding 240 mg/dL [54]. β-glucan emerges as a promising therapeutic agent, acting through multiple mechanisms to combat dyslipidemia. These mechanisms include reducing blood cholesterol levels, slowing bowel transit time, preventing constipation, increasing SCFA production, enhancing the growth of beneficial gut microbes, and reducing the risk of colorectal cancer.
One pivotal action of β-glucan is its ability to bind with bile acids, facilitating their excretion. This, in turn, reduces plasma cholesterol and bile acid levels. An increased elimination of bile acids promotes cholesterol metabolism in the liver, lowering lipid absorption and decreasing plasma cholesterol levels. Moreover, the fermentation of β-glucan in the large intestine produces acetate and butyrate, which inhibit cholesterol biosynthesis. β-glucan also participates in the degradation of LDL cholesterol. Recent research demonstrates that a daily intake of 3 g of β-glucan effectively lowers LDL cholesterol without significantly affecting high-density lipoproteins [55]. Furthermore, the high viscosity of β-glucan substantially impacts the absorption of cholesterol, fats, and other biomolecules in the gastrointestinal tract by increasing its viscosity. Incorporating fiber, particularly β-glucan-rich sources, into the diet offers a dual benefit: lowering serum cholesterol and reducing the intake of total fat, saturated fat, and dietary cholesterol [56].
The FDA has granted approval for the relationship between reduced blood cholesterol levels and increased DF absorption, specifically concerning psyllium and oat fiber. Notably, oat β-glucan supplementation has demonstrated a remarkable reduction in total cholesterol and LDL cholesterol by approximately 10% and 15% in a clinical trial involving slightly hypercholesterolemic individuals [57]. Further insights from studies involving oat-based diets in both rodents and humans suggest a link between increased acetate and butyrate levels and reduced cholesterol biosynthesis. Similarly, a comprehensive meta-analysis of 14 clinical studies reports a significant reduction in LDL cholesterol using barley β-glucan [58]. To delve into the mechanistic details, Ref. [59] explored hypercholesterolemic hamsters and observed that β-glucan from hull-less barley modulated the expression of key enzymes such as cholesterol 7-α hydroxylase (CYP7A1) and 3-hydroxy-3-methyl glutaryl-coenzyme A (HMG-CoA) reductase. This led to increased elimination of fecal lipids, resulting in decreased LDL cholesterol levels in the bloodstream. In vitro studies emphasize the significant impact of highland barley’s soluble DF in slowing cholesterol uptake, demonstrating a dose-dependent effect [60]. Human research, as exemplified by [61], indicates that the consumption of HB sprouts can enhance lipid metabolism and reduce the risk of cardiovascular disease, particularly in individuals with marginal cholesterol levels. In murine studies, the prolonged administration of highland barley β-glucan resulted in decreased serum total cholesterol, non-HDL cholesterol, LDL cholesterol, Lee’s index, and increased levels of SCFA. Similarly, mice subjected to higher doses of whole highland barley β-glucan exhibited reduced liver organ indices, abdominal fat, and liver lipid levels [62].
Notably, the molar mass of barley β-glucan plays a crucial role, with lower molar mass showing a 13% decrease in LDL cholesterol at a dosage of 5 g/day, while higher molar mass displays a 15% reduction [63]. An interesting dosage-dependent effect was observed in beverages fortified with β-glucan; the 5 g dose effectively lowered LDL and total cholesterol, whereas the 10 g dose did not [64], highlighting the significance of higher molar masses due to the solubility of solutions. A study was conducted with 29 hypercholesterolemia (early stage) children aged 6-14 years. This experiment assessed the performance of a packaged cereal containing β-glucan, including a minimal saturated fat and cholesterol intake, for lowering LDL cholesterol levels in children. In the initial four weeks, the children were kept on a diet of 3 g/d of β-glucan, and for the next four weeks, the children were provided with a substitute. In participants who were at least 80% in accordance (n = 18), total and soluble DF levels rose extensively (26.7%, p~0.01 and 30.8%, p~0.02, respectively), while LDL sank to an average of 5.3% (p~0.03). Individuals with a BMI under the median (25.7 kg/m2) had the greatest decrease in LDL levels(9.2%, p< 0.001) [65]. The β-glucan effects on the lipid profile, glycemia, and intestinal health (BELT) study looked into how 3 g of oat β-glucan per day affected plasma lipids, glucose levels in the fasting state, and intestinal sanity. The study took place in an 8 week, double-blind, placebo-controlled, cross-over randomized clinical experiment that enrolled 83 Italian adults who followed a Mediterranean diet and had mild hypercholesterolemia and narrow cardiovascular disease prospects. The BELT research shows an intermediate effectiveness of 3 g/day β-glucan supplementation in lowering LDL, total cholesterol, and non-HDL levels in moderate hypercholesterolemic patients despite being a Mediterranean culture [66].
Barley β-glucans of Mw, 290 kDa and 1350 kDa, at two distinct dosages, 3 and 5 g, were the subject of a pair of studies that examined the consequences of their use. When β-glucans were added to ready-to-eat cereal and fruit juice in the first research, both high and low MW were effective at lowering LDL and total cholesterol after six weeks of use in comparison to the control products without β-glucans. The second trial offered participants a breakfast of β-glucan-infused crepes, tortillas, oatmeal, or chips for five weeks. A substantial decrease in circulating total cholesterol was seen only with high β-glucan products, instead of the control goods made with wheat and rice. They concluded that the cholesterol-lowering benefits of β-glucans are determined by their physicochemical features (i.e., Mw) rather than their daily consumption [67]. Ref. [68] conducted a study where β-glucans were incorporated in a variety of dietary products, resulting in a noticeable reduction of LDL and TC. Another study that looked at administering 5 or 10 g of β-glucans to a beverage to supplement the typical diet for eight weeks found that β-glucans from oats instead of barley drastically dropped total cholesterol concentrations [64].
Research exploring the beneficial effects of β-glucans from various sources in dyslipidemia management is in its nascent stages, often involving small-scale clinical trials. The transition to large-scale trials for validation is essential. While the potential of β-glucans in dyslipidemia management is promising, significant questions persist. Detailed investigation into the influence of β-glucan properties, optimal dosage levels, and interactions with other dietary components is imperative. This knowledge will enable more precise and efficacious interventions for cholesterol management, ultimately reducing the risk of cardiovascular disease.
7.3. Mechanism of Immunomodulatory Effect of β-Glucan
The immune system is our body’s defense mechanism against invading pathogens and diseases. It comprises two primary components: the innate immune system and the adaptive immune system. The innate immune system acts rapidly and non-specifically against pathogens and includes cells like monocytes (macrophages), mast cells, dendritic cells, and complement proteins. These cells respond swiftly through mechanisms like phagocytosis, opsonization, and the release of cytokines, aiding in the differentiation of lymphocytes. On the other hand, the adaptive immune system, involving B and T lymphocytes and Natural Killer (NK) cells, provides a highly specific and long-lasting immune memory. B cells contribute to humoral immunity by producing antigen-specific antibodies, while T cells induce cytotoxic responses by secreting cytokines and chemokines.
A critical component in the realm of immunomodulation is β-glucan, a polysaccharide found in various sources, including cereals, fungi, and bacterial cells. This remarkable compound can serve as an adjuvant, generating immune signals. Its immunomodulatory effects are due to its recognition as a pathogen-associated molecular pattern (PAMP) by cell surface receptors known as pattern recognition receptors (PRRs). The innate immune system is the first line of defense, where β-glucan primarily exerts its influence. One key PRR that interacts with β-glucan is Dectin-1, which is expressed on myeloid cells like neutrophils, macrophages, and dendritic cells. Dectin-1 possesses an extracellular C-type lectin-like carbohydrate-recognizing domain (CRD), a transmembrane domain, and a cytosolic intracellular immune receptor tyrosine-based activation motif (ITAM). It has been demonstrated that Dectin-1 specifically recognizes a particular structural configuration of glucans, particularly β-glucans, with a minimum of seven glucan monomers in the backbone and one side chain branch. This recognition triggers downstream signaling that results in immune responses such as phagocytosis, respiratory burst, microbial cell destruction, and the secretion of cytokines [69].
Another receptor that interacts with β-glucan is Complement Receptor 3 (CR3), found on neutrophils, NK cells, and monocytes. CR3 plays a crucial role in the phagocytosis and opsonization of tumor cells through the binding of inactivated complement protein (iC3b). To activate CR3, β-glucan must bind to both β-1,3 glucan and iC3b. This interaction recruits NK cells and other phagocytic cells to the site of inflammation, stimulating phagocytosis and degranulation of opsonized complexes with iC3b, ultimately leading to the death of tumor cells. Toll-like receptors (TLRs) are another group of PRRs that recognize and bind to pathogen-associated molecular patterns (PAMPs). TLRs play a crucial role in activating both the innate and adaptive immune systems. Lactosylceramide, a glycosphingolipid found on the plasma membrane of immune cells, binds to β-glucan, triggering an immune response by inducing respiratory burst and secreting cytokines. β-glucan influences innate immunity and shapes the balance between T Helper (Th) 1 and Th2 cells. Maintaining this balance is essential, as an imbalance can lead to allergies and autoimmune diseases. β-glucan plays a role in shifting this equilibrium towards Th1 cells, reducing the risk of allergic diseases by altering the Th1/Th2 balance [70]. A study examined the impact of brewers’ yeast (1,3)-(1,6)- β-D-glucan ingestion on the frequency of common cold events in normal individuals. The current investigation showed that yeast β-D-glucan preparation improved the body’s ability to resist attacking bacterial and viral infections [71]. Grifola β–glucan can stimulate the growth of NK cells and lymphocytes in immunocompromised mice treated with cyclophosphamide (CTX). Grifola β–glucan may help safeguard mice from reduced immunity and bone marrow degradation resulting from cyclophosphamide by increasing the activity of kinases and transcription factors like p-Jak2/Jak2, p-Stat3/Stat3, and Socs3) in the Jak2/Stat3/Socs signaling cascade [72]. Another research sought to examine the influenced-by-time implications of oat beta-glucans affecting colon apoptosis and autophagy in the CD (Crohn’s disease) rat. Daily consumption of (high-molar-mass oat β-glucans) βGl and (high-molar-mass oat β-glucans) βGh dramatically decreased colitis through the time-varying alteration of autophagy and apoptosis, with βGl having a larger impact in apoptosis and βGh over autophagy. The pathway followed could be explained by the responsiveness of receptors like Dectin-1 and TLRs [73]. Taken together, β-glucan’s immunomodulatory effects are multifaceted and intricate, impacting various immune system components. Through interactions with PRRs, such as Dectin-1, CR3, and TLRs, β-glucan initiates a cascade of signaling events that activate immune cells and the secretion of various cytokines and chemokines. This intricate network of interactions and signaling pathways ultimately leads to enhanced immune responses, making β-glucan a promising agent for immune modulation and disease prevention. Future research should focus on optimizing the use of β-glucan as an immunomodulatory agent, exploring its potential in treating autoimmune diseases, and uncovering its interactions with other immune components. Additionally, more studies are needed to understand the specific mechanisms involved in β-glucan’s immunomodulation.
7.4. As a Potential Anticancer Molecule
Cancer, a highly intricate and multifaceted group of diseases characterized by uncontrolled cellular proliferation, poses formidable challenges in the realm of treatment. It stands as the second leading cause of mortality worldwide, responsible for approximately one in every six deaths [74]. Annually, there are nearly 19.3 million reported new cancer cases, resulting in approximately 10 million global fatalities [75]. The current gold-standard therapies, including surgery, chemotherapy, and radiotherapy, are associated with considerable side effects and yield a less-than-ideal prognosis.
β-glucan exerts potent anticarcinogenic effects by stimulating the host’s immune system, thereby thwarting oncogenesis and inhibiting tumor metastasis. Its anticancer impact stems from a multi-faceted approach involving direct tumor inhibition, immune system enhancement, and inherent anticancer properties. Various factors influence its antitumor efficacy, including the source, molecular structure, branching pattern, and chemical modifications of β-glucan. The remarkable repetitive structure of β-glucans enables them to bind to specific receptors on the membranes of immune cells, eliciting a robust anti-tumor response. This binding activates innate immunity, leading to accelerated antigen presentation, increased production of reactive oxygen species (ROS), enhanced phagocytosis, and the secretion of critical cytokines [76]. The secreted cytokines play a pivotal role in activating both B cells and T cells, thereby initiating adaptive immunity through humoral and cell-mediated immune responses, respectively.
To maximize the effectiveness of β-glucan’s anticarcinogenic properties, precise delivery throughout the body and the immune system is essential, underscoring the importance of understanding β-glucan trafficking [77]. Predominantly, oral administration serves as the primary mode of β-glucan delivery, although intraperitoneal (IP) and intravenous (IV) administration are also employed, albeit less frequently [77]. Following oral administration, β-glucans enter the proximal small intestine, where they are phagocytosed by intestinal epithelial cells or pinocytic microfold cells (M-cells). Subsequently, β-glucan is transported from the intestinal lumen to immune cells in Peyer’s patches. Upon encountering β-glucan, gastrointestinal macrophages migrate to the lymphatic system via the bloodstream [78]. In lymph nodes, β-glucan activates dendritic cells, which capture and imprison damaged tumor cells in the tumor microenvironment. This process leads to the differentiation and activation of antigen-specific CD4+ and CD8+ T-cells. Degraded fragments of β-glucan further activate neutrophils by binding with CR3 and modulating hematopoietic myeloid progenitors in the bone marrow. These events collectively trigger CD3-dependent cellular cytotoxicity (CR3-DCC) in proximity to opsonized iC3b-coated carcinogenic cells [79]. Orally administered β-glucan not only accelerates respiratory bursts and the rate of phagocytosis but also enhances the secretion of crucial cytokines, including interleukin-1 (IL-1), IL-6, and TNF-α within macrophages. Additionally, it regulates the acute phase of humoral immunity and augments the activity of lysozyme and ceruloplasmin in animal models [80]. These multifaceted mechanisms underpin the promising potential of β-glucan in combating cancer and stimulating immune responses.
The tumor microenvironment (TME) is an intricate network of elements, including blood vessels, extracellular matrix, malignant cells, and non-malignant immune cells. β-glucan, derived from fungi, possesses the remarkable ability to modulate the TME by regulating both innate and adaptive immune signals. This modulation begins with the activation of the host’s innate immunity through the binding of β-glucan to PRRs, triggering the differentiation, activation, and deployment of specific acquired adaptive immune cells, crucial for the host’s defense mechanism [81]. Macrophages, particularly those within the TME, significantly influence cancer prognosis. Notably, β-glucan’s interaction with Dectin-1 on tumor-associated macrophages (TAMs) accelerates the transition of M1 macrophages, enhancing antigen presentation and Th1 cytokine secretion [82]. Furthermore, β-glucan extracts and ganoderic acid from G. lucidum exhibit direct cytotoxic properties by activating M1 macrophages and subsequently destroying HepG2 cells [83]. The progression of the tumor is also influenced by Myeloid-derived suppressor cells (MDSCs), a distinct class of immune cells. Yeast-derived whole β-glucan particles (WGP) lead to a reduction in polymorphonuclear-MDSCs (PMN-MDSCs) through enhanced respiratory burst and apoptosis. β-glucan also impacts B lymphocyte differentiation and activation, promoting the development of humoral immune responses via the secretion of cytokines such as IL-6, IL-8, and TNF-α, mediated by the activation of NF-κB and AP-1 [84]. However, tumor cells can limit dendritic cell antigen presentation by secreting IL-10 and vascular endothelial growth factor (VEGF), evading the immune response [85]. Astragalus polysaccharide (APS) enhances dendritic cell activation by upregulating MHC-II, CD-80, and CD86 on dendritic cell surfaces, promoting synergistic interactions between T cells and dendritic cells [86]. Moreover, the TME orchestrates a process to stimulate angiogenesis to enhance oxygen and nutrient supply and eliminate metabolic waste, countering the hypoxic and acidic microenvironment. Furthermore, sulfated derivatives of glucan from Phellinus ribs (PRP-S1 and PRP-S2) inhibit tumor angiogenesis by reducing VEGF expression [87]. In an experiment, 20 participants who had terminal malignant tumors were administered a β-(1,3)/(1,6) D-glucan formulation and evaluated for tolerance and impact on hematopoiesis during chemotherapy. The findings suggest that β-glucan may improve hematopoiesis in cancer victims undergoing chemotherapy [88]. When paired with chemotherapy, Grifola β–glucan reduced the dimensions of breast, lung, and liver tumors in more than sixty percent of the subjects in contrast to chemotherapy isolated [89]. Research conducted revealed that oat β-D-glucan can hinder HTB-140 melanoma cells by escalating the stimulation of caspase-3/7 along with the surge of phosphatidylserine towards the extracellular face of the plasma membrane, which suggests the ordination of mitochondrial apoptosis [90]. A low-MW oat β-glucan showed firm activity of caspase-12 against A431 and Me45 cancer cell lines, leading to apoptosis and thereby showing its potential anticancerous activity [91].
In summary, β-glucan holds immense promise in the fight against cancer by stimulating the immune system, inhibiting tumor growth, and modulating the TME. However, there remain gaps in our understanding of the optimal delivery methods and the precise mechanisms underlying β-glucan’s multifaceted actions within the TME. Future research should focus on refining the delivery strategies, elucidating the specific immune pathways involved, and exploring potential combination therapies to maximize β-glucan’s anticancer potential.
7.5. Apoptosis
Apoptosis, a vital process in multicellular organism development, is a regulated form of cell death. It primarily eliminates damaged cells with irreversible DNA damage, contributing to the organism’s overall health. Key apoptotic hallmarks include chromatin condensation, cell shrinkage, membrane blebbing, and the loss of contact inhibition. Two primary pathways, the extrinsic and intrinsic pathways, orchestrate apoptosis. The extrinsic pathway relies on external signals, like TNF family death receptors (e.g., FasL/FasR), which activate caspase-8 and caspase-3, ultimately causing cell death [92]. In contrast, the intrinsic pathway involves factors such as the Bcl-2 protein family, with anti-apoptotic (e.g., Bcl-2) and pro-apoptotic (e.g., BAX) members. Dysregulation of these proteins can lead to cancer.
Various studies have elucidated the potent mechanisms by which different β-glucan isoforms or crude extracts can stimulate apoptosis, offering promising avenues for anti-cancer interventions. Notably, β-glucans sourced from bacterial origins have demonstrated their ability to induce apoptosis in SNU-C4 cells, as confirmed by TUNEL assay validation. This pro-apoptotic effect is underpinned by the upregulation of caspase-3, Bax, and the downregulation of Bcl-2. Furthermore, these β-glucans induce dose-dependent changes in cell morphology, the formation of apoptotic bodies, and chromatin condensation [93]. Hot water extracts from Chaga mushrooms have been shown to elevate apoptosis in HT-29 colon cancer cells. This effect is attributed to the modulation of key apoptotic regulators, including increased Bax and caspase-3 levels, coupled with a reduction in Bcl-2 expression [94]. In another line of investigation, β-glucans derived from Agaricus blazei Murill have exhibited a multifaceted mechanism of apoptosis induction. This includes the acceleration of cytochrome-C release, enhanced p38 MAPK activity, Bax translocation to mitochondria, and the activation of caspase-9. These molecular events culminate in apoptosis induction and contribute to reduced metastasis in a mouse tumor model [95]. Studies with extracts from Inonotus obliquus have unveiled their inhibitory effect on B16-F10 melanoma cells and human hepatoma HepG2 cells. This inhibition is associated with G0/G1 cell cycle arrest, apoptosis induction, and a reduction in key regulatory proteins like p53, p27, pRB, cyclin, and CDK [96]. Notably, extracts from Ganoderma lucidum have exhibited the ability to enhance apoptosis in ovarian cancer cells. This effect is achieved through the modulation of Akt, p53, and caspase-3 activation [97]. Breast cancer cells expressing estrogen receptors have been effectively hindered from proliferation through the treatment with β-1,3 glucan from Lentinus edodes (LNT). This anti-proliferative effect is corroborated using Western blotting, which revealed elevated p53 levels, phosphorylated ERK1/2, PARP-1, caspase-3, and reduced levels of p65, NF-kB, TERT, and MDM2 in tumor cells [98]. Furthermore, oat β-glucan has demonstrated its potential to induce apoptosis in human skin melanoma HTB-140 cells in a concentration-dependent manner. This action is marked by enhanced caspase 3/7 activation and phosphatidylserine accumulation on the cell surface, both of which drive apoptosis [90]. Lastly, extracellular β-glucans isolated from botryosphaeran and lasiodiplodan have been shown to induce oxidative stress in MCF-7 cells. The underlying mechanism of β-glucan-regulated apoptosis is supported by RT-PCR results, indicating the increased mRNA expression of forkhead transcription factor (FOXO-3a), p27, p53, AMP-activated protein kinase (AMPK), and a concomitant decrease in p70S6K [99].
The highlighted studies shed light on the multifaceted mechanisms by which various β-glucan isoforms and extracts induce apoptosis, holding significant promise for potential anti-cancer interventions. These mechanisms involve the modulation of key apoptotic regulators, the upregulation of pro-apoptotic factors like caspase-3 and Bax, and the downregulation of anti-apoptotic elements such as Bcl-2, as illustrated in Figure 5. The intrinsic and extrinsic pathways of apoptosis are influenced, and various cancer cell types are targeted, offering diverse strategies for combatting cancer. However, there are notable gaps and future perspectives in this area of research. While the mechanisms of action are becoming clearer, further studies are needed to unravel the specific signaling pathways and molecular interactions that underlie the effects of β-glucans on apoptosis. Additionally, more comprehensive in vivo studies are necessary to validate the potential clinical applications of these findings. The development of standardized β-glucan formulations and dosage regimens is also critical in translating this research into practical cancer therapies. Moreover, investigating the interplay between β-glucans and existing cancer treatments and exploring their potential synergy or antagonism is an important avenue for future research. Lastly, the safety profiles and long-term effects of β-glucans in human trials warrant careful examination. Overall, continued research in this field promises to expand our understanding of apoptosis regulation and improve cancer treatment strategies.
Figure 5.
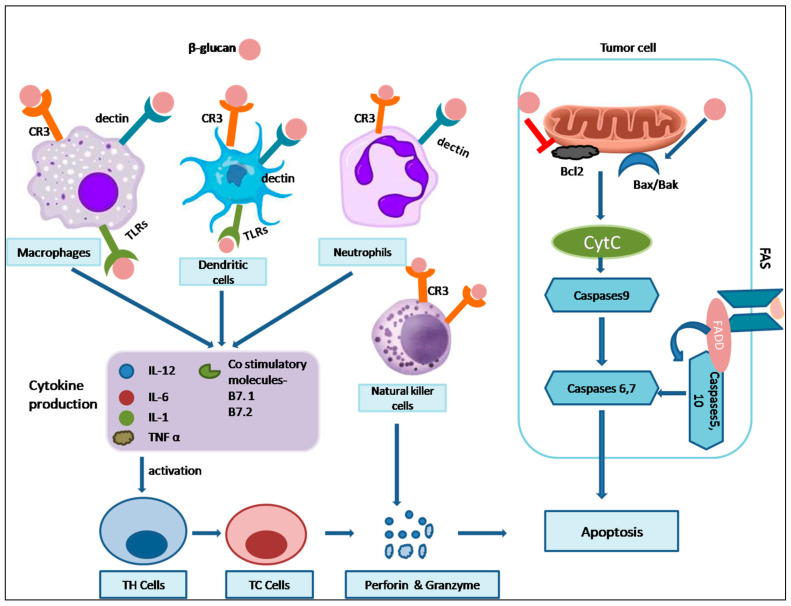
Open in a new tab
Immunomodulatory and proapoptotic effects of β-Glucan. β-glucan binds to the receptors such as dectin-1, CR-3, and TLRs present in immune cells like macrophages, dendritic cells, neutrophils, and natural killer cells to induce the production of cytokines and co-stimulatory molecules, including IL-12, IL-6, IL-1, TNF-α, B7.1, and B7.1 which results in the activation of Th and Tc cells. TH cells and Tc cells, along with natural killer cells, release perforin and granzyme, resulting in the apoptosis of cells. Also, β-glucan in the tumor cells binds to the FAS receptor, promotes Bax/Bak, and inhibits Bcl2, leading to the activation of caspases and, consequently, apoptosis of tumor cells. Abbreviations: TLRs: Toll-like receptors, CR3: complement receptor 3, IL: interleukin, TNF-α: tumor necrosis factor-α, TH: T helper cells, TC: T cytotoxic cells, Bcl2: B-cell lymphoma, Bax: Bcl associated X-protein, Bak: Bcl-2 antagonistic/killer, CytC: Cytochrome C, FAS: TNF Family death receptor, FADD: FAS associated protein and death domain.
7.6. β-Glucan in Gut Microbiota
The gut microbiota, often referred to as the “second brain” of the body, plays a pivotal role in maintaining overall human health. One of the critical functions of gut microbiota is the utilization of β-glucan, a DF, as a substrate. This fiber is essential for promoting healthy biological functions. Any disruption in the composition of gut microbes can lead to increased intestinal permeability, causing colonic inflammation that may contribute to the development of colon cancer. The human gut is home to an astounding population of over 100 trillion microbial organisms, collectively encoding a far greater number of genes than the human system itself. These microorganisms are indispensable for sustaining a healthy gut environment, actively participating in processes such as angiogenesis, modulation of signaling cascades, vitamin K biosynthesis, and the regulation of metabolic pathways [100].
The introduction of indigestible polysaccharides from various sources into the diet can enhance health by fostering the growth of beneficial microbial communities [101]. Once they escape digestion, these DFs reach the large intestine, where they undergo fermentation, producing SCFA, including acetate, propionate, and butyrate. Furthermore, the fermentation process yields compounds like indole, tryptophan derivatives, and secondary bile acids, all of which contribute to the advantageous effects observed [102]. In the processing of β-(1,3)-glucans, gut microbiota employs enzymes from the glycoside hydrolase family, such as β-(1,3) glucosidase (EC 3.2.1.58) and β-(1,3) glucanases (EC 3.2.1.6 and EC 3.2.1.39). β-(1,3)-glucanases break down internal glucosidic linkages, resulting in oligopolymers, while β-(1,3)-glucosidases act on the linkage at the non-reducing ends, releasing free glucose monomers [103]). The presence of carbohydrate-binding domains on a fraction of endo-acting β-(1,3)-glucanases enhances their binding capacity to water-insoluble compounds. To break down β-(1,6)-linked branched chains, another crucial enzyme, β-(1,6)-glucanase (EC 3.2.1.75), belonging to the GH30 family, is essential. The proliferation of epithelial cells may alter gut permeability and lead to inflammation, significantly affecting overall human health. Lactobacilli and Bifidobacteria species are particularly beneficial for gut health, and β-glucans from sources like oats and barley have been found to promote the growth of these species. [104] studied the effects of orally administered β-glucan on rabbit gut health and body growth, revealing improvements in body weight, total feed consumption, and antioxidant enzyme activity, indicating a modulation of anti-inflammatory responses and improved gut health. Furthermore, research involving the continuous administration of β-glucans from cereals to rats demonstrated an increase in the numbers of Lactobacillus and Bifidobacterium after 3, 6, and 7 weeks [105]. A clinical investigation evaluated the prebiotic ability of barley β-glucan in vivo using a randomized, double-blind, placebo-controlled design. Fifty-two healthy participants aged 39–70 had been allocated to have a cake with 0.75 g of barley β-glucan or a placebo every day for 30 days. The study found that taking a daily cake with barley β-glucan was tolerated effectively and had strong bifidogenic characteristics in healthy seniors [106].
The exploration of gut microbiota and its interaction with dietary β-glucan opens up several compelling avenues for future research. Understanding the specific enzymes and metabolic pathways that govern β-glucan processing by gut microbes is a fundamental research gap. This knowledge could optimize the dietary delivery of β-glucan for therapeutic purposes. Furthermore, investigating how gut microbiota composition can prevent colonic inflammation and related diseases, such as colon cancer, is a promising area. Identifying the key microorganisms and their functions in maintaining gut health is crucial for targeted interventions. The potential for DF to modulate the gut microbiota is an exciting research prospect, offering insights into how different fibers influence microbial communities and their metabolites, including SCFAs. Long-term studies on the effects of β-glucan supplementation in populations with metabolic syndrome are necessary to determine sustained benefits and potential side effects. The complex relationship between gut microbiota, β-glucan, and human health presents numerous research opportunities. Investigating microbial interactions, their impact on diseases, and long-term dietary interventions can enhance our understanding of gut health and personalized nutrition strategies.
8. Industrial Application of β-Glucan in Food, Medical and Cosmetics
In recent years, β-glucan has garnered significant attention due to its diverse applications within the industrial sectors of food, medicine, and cosmetics (Figure 6). Within the realm of food science, β-glucan has emerged as a functional ingredient, contributing to the enhancement of product textural properties, shelf stability, and nutritional profiles. Its remarkable immunomodulatory attributes have found relevance in the medical domain, potentially offering therapeutic avenues for an array of health conditions, including cardiovascular ailments and diabetes. Furthermore, in the cosmetic industry, β-glucan has been harnessed for its innate capacity to soothe and moisturize the skin, presenting a natural and efficacious component for skincare formulations. The multifaceted and dynamic utility of β-glucan underscores its significance as a valuable resource, fostering innovation and progress across these diverse industrial sectors. Further elaboration on recent advancements in each sector is presented in subsequent sections.
Figure 6.
Open in a new tab
Multifaceted applications of β-glucan in different industries.
8.1. Food and Beverage Industries
β-glucan, a multifunctional polysaccharide, is an essential ingredient in the food and beverage industry due to its remarkable thickening and gelation properties, as well as its ability to enhance solution solubility. This versatile compound improves the flavor, texture, and appearance of culinary delights such as ice creams, gravies, and salad dressings. Additionally, it acts as a calorie-reducing fat substitute, helping to meet the growing demand for healthier food options.
However, despite its promising applications, β-glucan presents significant technical challenges related to its flow behavior and gelling characteristics. These challenges can lead to limited yield, precipitation issues during beer storage, and delayed filtration in various industrial applications. To address these concerns, there is increasing interest in technologically advanced β-glucan texture enhancers, which have the potential to revolutionize its incorporation into different food matrices. Recent research highlights the effectiveness of hull-less barley β-glucan as a thickener and dietary fiber source in beverages and liquid products, supported by comprehensive rheological evaluations. Nonetheless, the high viscosity of β-glucans and their complex handling characteristics have restricted their widespread use. Therefore, it is crucial to understand the intricate relationship between β-glucan concentration and the properties it imparts to finished products.
β-glucan serves as a proficient thickening agent, fat substitute, emulsifier, and stabilizer for foams and emulsions. When combined with other hydrocolloids, it becomes a valuable source of soluble fiber in meat emulsions. Furthermore, β-glucan shows potential as a prebiotic in high-folate products. Recent research has examined the metabolic effects of functional bread enriched with β-glucans and resistant starch, assessing glycemic and insulin levels, as well as responses related to hunger and sensory attributes. Incorporating dates and Agaricus bisporus mushrooms into bread recipes enhances protein, iron content, nutritional quality, texture, sensory acceptance, and shelf life.
In the dairy sector, adding high molecular weight oat β-glucan to milk results in products with reduced calories and cholesterol. The phase behavior, rheological characteristics, and microstructure of these dairy products influence their flow behavior, particularly at higher concentrations. Yogurts enriched with pectin and β-glucan demonstrate enhanced proteolysis, reduced release of large peptides, and a higher proportion of free amino acids compared to counterparts made with starch or without β-glucan. The β-glucan content in food products, whether extracted from commercial sources or laboratories, varies significantly, with food matrix properties affecting the ideal concentration. For example, noodles may contain up to 10% β-glucan, soups about 2%, and meat emulsions range from 0.3% to 3%. Milk and dairy products typically contain around 2.5%, while bread can incorporate between 5.5% and 20%.
A study investigated the effects of hydroxypropyl methylcellulose, yeast β-glucan, and whey protein isolate in gluten-free bread made from rice starch-based formulas. Sensory evaluations revealed that yeast β-glucan-enhanced rice starch bread was well-received. Although the optimal β-glucan levels for different food groups remain unclear, research has established that electrostatic interactions between proteins and β-glucan occur during aggregation. Additionally, while β-glucans improve dough production due to their hydrocolloid nature, they can also affect the structural qualities of baked goods, leading to increased hardness, gumminess, and decreased cohesion and elasticity. Despite sensory differences, consumers generally prefer bread made with wheat flour over hulled barley whole grain flour. Nonetheless, these findings provide valuable insights for formulating functional bakery products.
Recent studies have primarily focused on β-glucan as an encapsulating agent for substances like fish oil and probiotics. As we progress into the twenty-first century, consumer demand for natural ingredients over synthetic ones is driving food processors to seek out dietary fibers and β-glucans as promising candidates in the growing nutraceutical market. β-glucan, derived from barley and oats, has been incorporated into two different soups. As predicted, freezing significantly impacted the molecular weight and sensory attributes of these soups, profoundly influencing their quality. Meanwhile, researchers successfully developed a barley extract beverage reminiscent of tea, with extraction temperatures ranging from 150 °C to 280 °C. The extracted materials were analyzed for their physical and chemical properties.
8.2. Cosmetic Industries
β-glucan has found widespread application in the cosmetics and personal care product industry. Scientific studies have highlighted its numerous benefits in skincare. Derived from sources such as mushrooms, cereals, and microorganisms, β-glucan exhibits soothing, moisturizing, and anti-irritant properties. Its skin-regenerating capabilities are well-documented, making it a valuable addition to cosmetic formulations. Notably, β-glucan is recognized for its ability to hydrate the skin and mucosa, revitalizing them to an optimal condition. Researchers have also harnessed β-glucan’s exceptional moisture retention properties for innovative applications.
The efficacy of natural β-glucan from various sources has been studied in relation to improving overall skin health. It extends its significance beyond skincare, being utilized to address a range of concerns, including skin aging, vaccine production, eye ulcer treatment, and dermatological procedures. Its presence in cosmetic products—such as sunscreens, creams, and powders—is supported by its roles in stimulating collagen production, reducing fine wrinkles, and alleviating conditions like eczema. Additionally, β-glucan shows promise for wound healing and has the potential to mitigate oxidative tissue damage, making it a candidate for wound dressing applications. The combination of β-glucan with chitosan opens up new possibilities for therapeutic wound dressings. Research also indicates that β-glucan may help reverse skin aging and minimize wrinkles, showcasing its versatility and appeal in the cosmetic industry.
From its potential as an eye drop derived from mushrooms to its ability to strengthen the immune system and scavenge free radicals, the applications of β-glucan are extensive. Studies have shown that β-glucan derived from baker's yeast may also have promising prospects in cosmetics and therapeutic products. Evidence suggests that CM-glucan stimulates keratinocyte growth, enhances skin resilience against damage from detergents, and accelerates the regeneration of the stratum corneum. Oat β-glucan, such as “Avenacare,” is a preferred ingredient in personal care and cosmetic products due to its calming, hydrating, and anti-irritant properties.
Despite the extensive research highlighting the diverse applications and benefits of β-glucan in the cosmetics and personal care industry, several research gaps and opportunities for future exploration remain. One key area for investigation is optimizing β-glucan’s extraction methods from various natural sources. Improved techniques for maximizing yield and purity could enhance its utility in cosmetic formulations. Although evidence supports its effectiveness in skin regeneration, further studies are needed to clarify the underlying mechanisms and determine the precise dosages required for optimal results. The role of β-glucan in addressing specific skin conditions, such as eczema and skin aging, could be a central focus for future research, including clinical trials to establish its practical efficacy.
Moreover, understanding the synergistic effects of β-glucan when combined with other skincare ingredients could lead to the development of more impactful formulations. Research on the long-term effects of β-glucan in skincare products and its interaction with various skin types and conditions is essential. While the potential use of β-glucan in wound dressings is promising, further studies could explore the development of innovative wound care products that effectively leverage its properties. Investigating the application of β-glucan in hair care products and its benefits for scalp health is another exciting avenue worth exploring.
Rigorous safety and toxicity assessments are necessary to establish a comprehensive scientific basis for incorporating β-glucan into cosmetics and personal care products. This includes examining any potential side effects or sensitivities across different populations. By addressing these research gaps, we can unlock the full potential of β-glucan in enhancing skincare and personal care products.
8.3. Medical Industries
The significance of β-glucans in the medical industry is immense. Clinical trials dating back to the 1980s introduced the concept of using fungal β-glucans as adjuvant therapies for cancer, paving the way for new treatment approaches. In the ever-evolving landscape of medical research, β-glucans have emerged as a beacon of hope in the ongoing battle against cancer. These remarkable compounds, derived from sources such as lentinan, D-fraction, and schizophyllan, have garnered considerable attention for their potential to revolutionize cancer therapy. As one of the leading causes of death worldwide, cancer remains a challenge for conventional treatment methods, including radiation therapy, chemotherapy, and surgical resection. However, β-glucans offer a transformative alternative. These natural agents have demonstrated potent anticancer effects against various types of cancer, including gastrointestinal, breast, and lung cancers. They work by inducing cytotoxicity in cancer cells, inhibiting proliferation, and promoting apoptosis through complex molecular pathways. The profound potential of β-glucans in medical science provides a promising glimpse into a future where cancer treatment is more effective, safer, and less likely to entail the horrors of metastasis and recurrence. Ongoing research heralds the dawn of a new era in cancer therapy, emphasizing the vital role of β-glucans as a catalyst for innovation within the medical field. Moreover, the impact of β-glucans extends beyond direct cancer treatment. Their immunomodulatory properties initiate a cascade of immune responses, including the secretion of pro-inflammatory and anti-inflammatory cytokines and the activation of natural killer (NK) cells, T cells, and macrophages, all of which actively engage with tumor cells. Recent studies have explored the immunological mechanisms of various β-glucans, highlighting their ability to enhance phagocytosis and IL-2 release, as well as to inhibit cancer growth. Additionally, these compounds have shown promise in mitigating the effects of experimental infections. Recent research has identified β-glucans as having significant potential for use in the treatment of COVID-19, caused by the SARS-CoV-2 virus. β-glucans have been reported to boost immunity and alleviate COVID-19 symptoms when taken as preventive oral supplements. They can stimulate several immune pathways, including antibody production, and promote an immunomodulatory effect, improving the cellular responses involved in the infectious process and reducing the inflammatory cytokines associated with COVID-19. Specifically, β-glucans from Lentinula edodes (lentinan) have been studied for their ability to reduce lung damage caused by pneumonia associated with SARS-CoV-2 due to their cytoprotective effects. Although there has been limited research on autoimmune disorders, an extract from Agaricus blazei was tested in inflammatory bowel disease with modest results regarding inflammatory cytokines and clinical symptoms. Furthermore, β-glucans expedite wound healing by enhancing macrophage infiltration, promoting tissue granulation, collagen synthesis, and skin re-epithelialization. A clinical trial found that using soluble yeast β-1,3-glucan allowed 59% of all ulcers to heal by week 12, compared to just 37% in the control group. It is hypothesized that the gel-forming properties of β-glucans play a major role in their ability to regulate bile acid and cholesterol metabolism. By reducing the intestinal absorption of dietary cholesterol and blocking bile acid reabsorption, β-glucans also increase the requirement for bile acids to be synthesized from cholesterol catabolism, resulting in lower blood cholesterol levels. Recently, the regulation of cholesterol homeostasis has been linked to the effect of β-glucans on gut microbiota modulation, particularly on bacterial species that influence bile acid metabolism and the generation of short-chain fatty acids. Consumption of oats or oat-derived β-glucans has been associated with reduced LDL cholesterol levels in several randomized controlled studies and subsequent meta-analyses. The multifaceted potential of β-glucans in the medical industry presents a fertile ground for future research. While significant progress has been made in understanding their role in cancer therapy, several research gaps remain. There is a need for further clinical trials and mechanistic studies to determine the optimal dosage, administration methods, and long-term effects of β-glucans in cancer treatment. Additionally, exploring their synergistic potential in combination with conventional therapies could enhance their clinical utility. Furthermore, the immunomodulatory properties of β-glucans, particularly in the context of infectious diseases, warrant further investigation.
9. β-Glucan’s Global Trade Potential
Barley and oats are well-known sources of plant-derived β-glucan, with barley containing a higher β-glucan content than oats and yeast. This high β-glucan content, coupled with an impressive extraction rate of up to 80%, makes barley an efficient and cost-effective option for obtaining this vital dietary fiber. As the demand for natural, plant-based ingredients continues to grow, barley and oats are ideally positioned to cater to health-conscious consumers seeking natural products. This trend reflects an increasing awareness of the health benefits associated with these grains, making them attractive options for individuals looking to incorporate more plant-derived nutrients into their diets. Consequently, cereals—especially barley and oats—are widely regarded as the preferred commercial sources of β-glucan.
Commercial products in the market show considerable variations in β-glucan isolation processes, concentration, molecular weight, water-holding capacity, and viscosity. Researchers have made various efforts to incorporate β-glucan into diverse food products, including those made from cereals, meat, and dairy. This highlights the multifaceted applications of β-glucan in the global market.
The global market for food-grade β-glucan is poised for substantial growth. Current data from Market and Markets.com indicates that the majority of β-glucan manufacturing companies are located in the United States (e.g., Super Β-glucan, Kemin Industries, Merck, International Flavors & Fragrances Inc.) and the European Union (e.g., Tate and Lyle PLC, Kerry Group PLC, Ohly GmbH). This distribution emphasizes the potential for market expansion in other regions, suggesting promising international development opportunities within the β-glucan industry.
The β-glucan market is divided into specific segments, including cereals and grains, yeast, mushrooms, and seaweed. The cereals and grains sector is expected to dominate, with revenues surpassing USD 229 million in 2023, and is projected to continue growing at a compound annual growth rate (CAGR) of 8.1%. This sector’s prominence is due to the long-standing practice of extracting β-glucan from these easily produced and processed sources.
The current global market valuation for β-glucan, derived from various sources, is approximately USD 500 million. It is expected to experience robust growth, surpassing USD 700 million by 2028, with a CAGR of 7.9%. Specifically, the North American β-glucan market is predicted to reach USD 272.7 million by 2028, displaying a CAGR of 8.3%, while the European Union is expected to achieve a market size of approximately USD 200 million by 2028, with a CAGR of 8.1%.
The expansion of the Asia-Pacific market is driven by health-conscious consumers and the abundant supply of cereals and grains, the primary sources of β-glucan. The growth of the global β-glucan market is largely propelled by rising consumer health awareness and increasing health-related expenditures. Additionally, the diverse applications of β-glucan across various industries contribute to market growth. However, challenges such as regulatory inconsistencies and price volatility of raw materials may hinder this growth. Nevertheless, the market stands to benefit from untapped applications for β-glucan, and the growing demand for natural and plant-based ingredients presents further opportunities for expansion.
10. Conclusions and Future Perspective
In conclusion, β-glucan, a complex polysaccharide present in various natural sources such as cereals, mushrooms, and algae, holds significant promise for nutrition, health, and industry. Recent research has illuminated its structural characteristics, sources, bioavailability, and biosynthetic pathways, yielding valuable insights into its potential applications. In this overview, we summarize the key findings and outline future directions for the multifaceted field of β-glucan research.
Notably, researchers have uncovered critical insights into the structural characteristics of β-glucan and the genetic factors involved in its synthesis. Genes like CslF6 have been identified as essential for β-glucan production, thereby enhancing our understanding of the biosynthetic processes involved. The dynamic changes in β-glucan levels during plant growth and development add complexity to this field and require further investigation.
β-glucan’s diverse structural properties and functional attributes have important implications for human health. It has been shown to potentially reduce cholesterol levels and modulate immune responses, making it a valuable dietary component. Research into its hypoglycemic effects and potential for weight management further emphasizes its relevance in the context of health.
The future of β-glucan research holds exciting prospects. Here, we highlight seven key areas for future exploration:
1. **Genetic and Epigenetic Regulation**: Understanding the regulatory mechanisms controlling β-glucan production in plants, at both genetic and epigenetic levels, will deepen our knowledge of plant biology. Advances in genome editing technologies, such as CRISPR/Cas9, could allow for precise engineering of β-glucan content and structure in crops, enhancing their nutritional value and industrial applications.
2. **Impact on Plant Growth and Development**: Investigating how manipulating β-glucan levels affects plant growth, development, and immunity will contribute to a broader understanding of plant biology and its applications in agriculture.
3. **Bioavailability and Gut Interactions**: Research into the bioavailability of β-glucan and its interactions with gut bacteria is necessary to optimize its therapeutic potential. These insights will be crucial for enhancing the health benefits associated with consuming β-glucan.
4. **Personalized Nutrition**: Exploring personalized β-glucan interventions, elucidating specific molecular pathways, and developing innovative β-glucan-enriched food products can optimize its use for glycemic control, cholesterol management, and metabolic health tailored to individuals with diverse dietary and genetic profiles.
5. **Clinical Trials and Medical Applications**: Further clinical trials are essential to validate the therapeutic potential of β-glucans for applications such as cancer treatment, immunomodulation, and managing metabolic syndrome. These trials will help translate research findings into practical treatments.
6. **Sustainability and Green Chemistry**: Integrating sustainable practices and green chemistry principles into β-glucan production and purification processes will improve environmental sustainability. This includes exploring innovative approaches such as nanotechnology and advanced chromatographic techniques.
7. **Advanced Food and Cosmetic Applications**: Ongoing research into optimizing β-glucan concentrations in food products, assessing long-term health effects, and investigating advanced processing techniques is essential. In the cosmetic industry, further exploration of extraction methods, mechanisms of action for skin conditions, and synergistic effects with other skincare ingredients can lead to innovative developments.
The dynamic and multidisciplinary field of β-glucan research continues to offer numerous opportunities in nutrition, health, and industry. As we delve deeper into its molecular mechanisms, health benefits, and industrial applications, β-glucan is set to play a pivotal role in enhancing human well-being while contributing to sustainable agricultural practices, food production, and healthcare solutions. Ongoing and future studies in this area will uncover the vast potential of β-glucan in shaping a healthier and more sustainable future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16060900/s1, Table S1: Genes involved in the synthesis of β-Glucan in yeast; Table S2: Different extraction methodologies developed and used to extract protein from the different sources of β-glucan. References [131,132,133,134,135,136,137,138,139,140,141,142] are cited in Supplementary File.
nutrients-16-00900-s001.zip (235.8KB, zip)
Author Contributions
A.S.: Methodology, investigation, data curation, formal analysis, writing—original draft. O.P.G.: Methodology, investigation, data curation, formal analysis, writing—original draft. V.S.: Methodology, investigation, data curation, formal analysis. A.K.: Methodology, investigation, data curation, formal analysis. N.P.: Methodology, investigation, data curation, formal analysis. N.M.: Methodology, investigation, data curation, formal analysis. A.: Methodology, investigation, data curation, formal analysis. D.K.: formal analysis, writing—original draft. O.V.: investigation, writing—original draft. J.S.: writing—original draft. L.K.: writing—original draft. C.L.: writing—original draft. G.S.: editing, supervision. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The authors are grateful to the Indian Council of Agricultural Research, the Department of Agricultural Research and Education, Government of India for providing financial help.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instruction,s or products referred to in the content.
References
1.Astiz V., Guardianelli L.M., Salinas M.V., Brites C., Puppo M.C. High β-Glucans oats for healthy wheat breads: Physicochemical properties of dough and breads. Foods. 2022;12:170. doi: 10.3390/foods12010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
2.Tharifkhan S.A., Perumal A.B., Elumalai A.K., Moses J.A., Anandharamakrishnan C. Improvement of nutrient bioavailability in millets: Emphasis on the application of enzymes. J. Sci. Food Agric. 2021;101:4869–4878. doi: 10.1002/jsfa.11228. [DOI] [PubMed] [Google Scholar]
3.Lante A., Canazza E. Insight on Extraction and Preservation of Biological Activity of Cereal β-D-Glucans. Appl. Sci. 2023;13:11080. doi: 10.3390/app131911080. [DOI] [Google Scholar]
4.Mykhalevych A., Polishchuk G., Nassar K., Osmak T., Buniowska-Olejnik M. β-Glucan as a techno-functional ingredient in dairy and milk-based products—A review. Molecules. 2022;27:6313. doi: 10.3390/molecules27196313. [DOI] [PMC free article] [PubMed] [Google Scholar]
5.Burton R.A., Collins H.M., Kibble N.A.J., Smith J.A., Shirley N.J., Jobling S.A., Henderson M., Singh R.R., Pettolino F., Wilson S.M., et al. Over-expression of specific HvCslF cellulose synthase-like genes in transgenic barley increases the levels of cell wall (1,3; 1,4)-β-d-glucans and alters their fine structure. Plant Biotechnol. J. 2011;9:117–135. doi: 10.1111/j.1467-7652.2010.00532.x. [DOI] [PubMed] [Google Scholar]
6.Tiwari U., Cummins E. Meta-analysis of the effect of β-glucan intake on blood cholesterol and glucose levels. Nutrition. 2011;27:1008–1016. doi: 10.1016/j.nut.2010.11.006. [DOI] [PubMed] [Google Scholar]
7.Cerletti C., Esposito S., Iacoviello L. Edible mushrooms and beta-glucans: Impact on human health. Nutrients. 2021;13:2195. doi: 10.3390/nu13072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
8.Jedinak A., Dudhgaonkar S., Wu Q.-L., Simon J., Sliva D. Anti-inflammatory activity of edible oyster mushroom is mediated through the inhibition of NF-κB and AP-1 signaling. Nutr. J. 2011;10:52. doi: 10.1186/1475-2891-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
9.Prins A., Shewry P., Lovegrove A. Analysis of mixed linkage β-glucan content and structure in different wheat flour milling fractions. J. Cereal Sci. 2023;113:103753. doi: 10.1016/j.jcs.2023.103753. [DOI] [Google Scholar]
10.Mejía S.M.V., De Francisco A., Bohrer B. A comprehensive review on cereal β-glucan: Extraction, characterization, causes of degradation, and food application. Crit. Rev. Food Sci. Nutr. 2020;60:3693–3704. doi: 10.1080/10408398.2019.1706444. [DOI] [PubMed] [Google Scholar]
11.Leković S., Marković S., Minić Đ., Todosijević J., Torbica A., Đukić N. B-glucan content variability in seed of barley cultivars. Kragujev. J. Sci. 2023;45:111–120. doi: 10.5937/KgJSci2345111L. [DOI] [Google Scholar]
12.Hamad S.A., Mustafa A.I., Magboul B.I., Qasem A.A., Ahmed I.A.M. Nutritional quality of raw and cooked flours of a high β-glucan sorghum inbred line. J. Cereal Sci. 2019;90:102857. doi: 10.1016/j.jcs.2019.102857. [DOI] [Google Scholar]
13.Ishibashi K.-I., Miura N.N., Adachi Y., Ohno N., Yadomae T. Relationship between solubility of grifolan, a fungal 1,3-β-D-glucan, and production of tumor necrosis factor by macrophages in vitro. Biosci. Biotechnol. Biochem. 2001;65:1993–2000. doi: 10.1271/bbb.65.1993. [DOI] [PubMed] [Google Scholar]
14.Zhang Y., Kong H., Fang Y., Nishinari K., Phillips G.O. Schizophyllan: A review on its structure, properties, bioactivities and recent developments. Bioact. Carbohydr. Diet. Fibre. 2013;1:53–71. doi: 10.1016/j.bcdf.2013.01.002. [DOI] [Google Scholar]
15.Ooi V.E., Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000;7:715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
16.Frasnelli M.E., Tarussio D., Chobaz-Péclat V., Busso N., So A. TLR2 modulates inflammation in zymosan-induced arthritis in mice. Arthritis Res. Ther. 2005;7:R370. doi: 10.1186/ar1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
17.Skjermo J., Størseth T.R., Hansen K., Handå A., Øie G. Evaluation of β-(1→ 3, 1→ 6)-glucans and High-M alginate used as immunostimulatory dietary supplement during first feeding and weaning of Atlantic cod (Gadus morhua L.) Aquaculture. 2006;261:1088–1101. doi: 10.1016/j.aquaculture.2006.07.035. [DOI] [Google Scholar]
18.Kataoka K., Muta T., Yamazaki S., Takeshige K. Activation of macrophages by linear (1→ 3)-β-d-glucans: Implications for the recognition of fungi by innate immunity. J. Biol. Chem. 2002;277:36825–36831. doi: 10.1074/jbc.M206756200. [DOI] [PubMed] [Google Scholar]
19.Zhang J., Yan L., Liu M., Guo G., Wu B. Analysis of β-d-glucan biosynthetic genes in oat reveals glucan synthesis regulation by light. Ann. Bot. 2021;127:371–380. doi: 10.1093/aob/mcaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
20.Geng L., Li M., Xie S., Wu D., Ye L., Zhang G. Identification of genetic loci and candidate genes related to β-glucan content in barley grain by genome-wide association study in International Barley Core Selected Collection. Mol. Breed. 2021;41:6. doi: 10.1007/s11032-020-01199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
21.Geng L., He X., Ye L., Zhang G. Identification of the genes associated with β-glucan synthesis and accumulation during grain development in barley. Food Chem. Mol. Sci. 2022;5:100136. doi: 10.1016/j.fochms.2022.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
22.Nemeth C., Freeman J., Jones H.D., Sparks C., Pellny T.K., Wilkinson M.D., Dunwell J., Andersson A.A., Åman P., Guillon F., et al. Down-regulation of the CSLF6 gene results in decreased (1,3; 1,4)-β-D-glucan in endosperm of wheat. Plant Physiol. 2010;152:1209–1218. doi: 10.1104/pp.109.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
23.Garcia-Gimenez G., Barakate A., Smith P., Stephens J., Khor S.F., Doblin M.S., Hao P., Bacic A., Fincher G.B., Burton R.A., et al. Targeted mutation of barley (1,3; 1,4)-β-glucan synthases reveals complex relationships between the storage and cell wall polysaccharide content. Plant J. 2020;104:1009–1022. doi: 10.1111/tpj.14977. [DOI] [PubMed] [Google Scholar]
24.Burton R.A., Fincher G.B. Current challenges in cell wall biology in the cereals and grasses. Front. Plant Sci. 2012;3:130. doi: 10.3389/fpls.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
25.Taketa S., Yuo T., Tonooka T., Tsumuraya Y., Inagaki Y., Haruyama N., Larroque O., Jobling S.A. Functional characterization of barley betaglucanless mutants demonstrates a unique role for CslF6 in (1,3; 1,4)-β-D-glucan biosynthesis. J. Exp. Bot. 2012;63:381–392. doi: 10.1093/jxb/err285. [DOI] [PMC free article] [PubMed] [Google Scholar]
26.Bain M., van de Meene A., Costa R., Doblin M.S. Characterisation of cellulose synthase like F6 (CslF6) mutants shows altered carbon metabolism in β-D-(1,3; 1,4)-glucan deficient grain in Brachypodium distachyon. Front. Plant Sci. 2021;11:602850. doi: 10.3389/fpls.2020.602850. [DOI] [PMC free article] [PubMed] [Google Scholar]
27.Vega-Sánchez M.E., Loqué D., Lao J., Catena M., Verhertbruggen Y., Herter T., Yang F., Harholt J., Ebert B., Baidoo E.E.K., et al. Engineering temporal accumulation of a low recalcitrance polysaccharide leads to increased C6 sugar content in plant cell walls. Plant Biotechnol. J. 2015;13:903–914. doi: 10.1111/pbi.12326. [DOI] [PubMed] [Google Scholar]
28.Burton R.A., Wilson S.M., Hrmova M., Harvey A.J., Shirley N.J., Medhurst A., Stone B.A., Newbigin E.J., Bacic A., Fincher G.B. Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3; 1,4)-ß-D-glucans. Science. 2006;311:1940–1942. doi: 10.1126/science.1122975. [DOI] [PubMed] [Google Scholar]
29.Jobling S.A. Membrane pore architecture of the CslF6 protein controls (1-3, 1-4)-β-glucan structure. Sci. Adv. 2015;1:e1500069. doi: 10.1126/sciadv.1500069. [DOI] [PMC free article] [PubMed] [Google Scholar]
30.Buckeridge M.S., Rayon C., Urbanowicz B., Tiné M.A.S., Carpita N.C. Mixed linkage (1→ 3),(1→ 4)-β-d-glucans of grasses. Cereal Chem. 2004;81:115–127. doi: 10.1094/CCHEM.2004.81.1.115. [DOI] [Google Scholar]
31.Marcotuli I., Colasuonno P., Blanco A., Gadaleta A., Ilaria M., Pasqualina C., Antonio B., Agata G. Expression analysis of cellulose synthase-like genes in durum wheat. Sci. Rep. 2018;8:15675. doi: 10.1038/s41598-018-34013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
32.Cabib E., Kang M.S. Methods in Enzymology. Volume 138. Academic Press; Cambridge, MA, USA: 1987. Fungal 1, 3-β-glucan synthase; pp. 637–642. [DOI] [PubMed] [Google Scholar]
33.Venkatachalam G., Arumugam S., Doble M. Industrial production and applications of α/β linear and branched glucans. Indian Chem. Eng. 2021;63:533–547. doi: 10.1080/00194506.2020.1798820. [DOI] [Google Scholar]
34.Zhu F., Du B., Xu B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016;52:275–288. doi: 10.1016/j.foodhyd.2015.07.003. [DOI] [Google Scholar]
35.Hu X., Zhao J., Zhao Q., Zheng J. Structure and Characteristic of β-Glucan in Cereal: A Review. J. Food Process. Preserv. 2015;39:3145–3153. doi: 10.1111/jfpp.12384. [DOI] [Google Scholar]
36.Temelli F., Bansema C., Stobbe K. Development of an orange-flavored barley β-glucan beverage. Cereal Chem. 2004;81:499–503. doi: 10.1094/CCHEM.2004.81.4.499. [DOI] [Google Scholar]
37.Zhu F., Du B., Bian Z., Xu B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015;41:165–173. doi: 10.1016/j.jfca.2015.01.019. [DOI] [Google Scholar]
38.Han C., Ma M., Li M., Sun Q. Further interpretation of the underlying causes of the strengthening effect of alkali on gluten and noodle quality: Studies on gluten, gliadin, and glutenin. Food Hydrocoll. 2020;103:105661. doi: 10.1016/j.foodhyd.2020.105661. [DOI] [Google Scholar]
39.Skendi A., Papageorgiou M. Introduction in wheat and breadmaking. In: Galanakis C.M., editor. Trends in Wheat and Bread Making. Academic Press; Cambridge, MA, USA: 2021. pp. 1–27. [DOI] [Google Scholar]
40.Fazio A., La Torre C., Caroleo M.C., Caputo P., Plastina P., Cione E. Isolation and purification of glucans from an italian cultivar of Ziziphus jujuba Mill. and in vitro effect on skin repair. Molecules. 2020;25:968. doi: 10.3390/molecules25040968. [DOI] [PMC free article] [PubMed] [Google Scholar]
41.Meng X., Liang H., Luo L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016;424:30–41. doi: 10.1016/j.carres.2016.02.008. [DOI] [PubMed] [Google Scholar]
42.Zhang H., Zhang N., Xiong Z., Wang G., Xia Y., Lai P., Ai L. Structural characterization and rheological properties of β-D-glucan from hull-less barley (Hordeum vulgare L. var. nudum Hook. f.) Phytochemistry. 2018;155:155–163. doi: 10.1016/j.phytochem.2018.08.004. [DOI] [PubMed] [Google Scholar]
43.Izydorczyk M.S., Dexter J.E. Barley β-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products—A Review. Food Res. Int. 2008;41:850–868. doi: 10.1016/j.foodres.2008.04.001. [DOI] [Google Scholar]
44.Ohno N., Miura N.N., Chiba N., Adachi Y., Yadomae T. Comparison of the immunopharmacological activities of triple and single-helical schizophyllan in mice. Biol. Pharm. Bull. 1995;18:1242–1247. doi: 10.1248/bpb.18.1242. [DOI] [PubMed] [Google Scholar]
45.Suárez E.R., Syvitski R., Kralovec J.A., Noseda M.D., Barrow C.J., Ewart H.S., Lumsden M.D., Grindley T.B. Immunostimulatory polysaccharides from Chlorella p yrenoidosa. A new galactofuranan. Measurement of molecular weight and molecular weight dispersion by DOSY NMR. Biomacromolecules. 2006;7:2368–2376. doi: 10.1021/bm060365x. [DOI] [PubMed] [Google Scholar]
46.Rice P.J., Adams E.L., Ozment-Skelton T., Gonzalez A.J., Goldman M.P., Lockhart B.E., Barker L.A., Breuel K.F., DePonti W.K., Kalbfleisch J.H., et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. 2005;314:1079–1086. doi: 10.1124/jpet.105.085415. [DOI] [PubMed] [Google Scholar]
47.Nakamura S., Tanabe K., Morita S., Hamaguchi N., Shimura F., Oku T. Metabolism and bioavailability of newly developed dietary fiber materials, resistant glucan and hydrogenated resistant glucan, in rats and humans. Nutr. Metab. 2016;13:13. doi: 10.1186/s12986-016-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
48.De Souza N.L., Bartz J., Zavareze E.D.R., Oliveira P., da Silva W.S.V., Alves G.H., Dias A.R.G. Functional, thermal and rheological properties of oat β-glucan modified by acetylation. Food Chem. 2015;178:243–250. doi: 10.1016/j.foodchem.2015.01.079. [DOI] [PubMed] [Google Scholar]
49.Chakraborty S., Rajeswari V.D. Biomedical aspects of beta-glucan on glucose metabolism and its role on primary gene PIK3R1. J. Funct. Foods. 2022;99:105296. doi: 10.1016/j.jff.2022.105296. [DOI] [Google Scholar]
50.Thondre P.S., Henry C.J.K. High-molecular-weight barley β-glucan in chapatis (unleavened Indian flatbread) lowers glycemic index. Nutr. Res. 2009;29:480–486. doi: 10.1016/j.nutres.2009.07.003. [DOI] [PubMed] [Google Scholar]
51.Lobato R.V., Silva V.O., Andrade E.F., Orlando D.R., Zangeronimo M.G., de Souza R.V., Pereira L.J. Metabolic effects of β-glucans (Saccharomyces cerevisae) per os administration in rats with streptozotocin-induced diabetes. Nutr. Hosp. 2015;32:256–264. doi: 10.3305/nh.2015.32.1.9013. [DOI] [PubMed] [Google Scholar]
52.Dong J., Cai F., Shen R., Liu Y. Hypoglycaemic effects and inhibitory effect on intestinal disaccharidases of oat beta-glucan in streptozotocin-induced diabetic mice. Food Chem. 2011;129:1066–1071. doi: 10.1016/j.foodchem.2011.05.076. [DOI] [PubMed] [Google Scholar]
53.AbuMweis S., Thandapilly S.J., Storsley J., Ames N. Effect of barley β-glucan on postprandial glycaemic response in the healthy human population: A meta-analysis of randomized controlled trials. J. Funct. Foods. 2016;27:329–342. doi: 10.1016/j.jff.2016.08.057. [DOI] [Google Scholar]
54.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
55.Kumar D., Narwal S., Virani S., Verma R.P.S., Gyawali S., Singh G.P. Barley grain beta glucan enrichment: Status and opportunities. In: Gupta O.P., Pandey V., Narwal S., Sharma P., Ram S., Singh G.P., editors. Wheat and Barley Grain Biofortification. Woodhead Publishing; Sawston, UK: 2020. pp. 295–308. [Google Scholar]
56.Kim S.Y., Song H.J., Lee Y.Y., Cho K.-H., Roh Y.K. Biomedical issues of dietary fiber β-glucan. J. Korean Med. Sci. 2006;21:781–789. doi: 10.3346/jkms.2006.21.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
57.Behall K.M., Scholfield D.J., Hallfrisch J. Effect of beta-glucan level in oat fiber extracts on blood lipids in men and women. J. Am. Coll. Nutr. 1997;16:46–51. doi: 10.1080/07315724.1997.10718648. [DOI] [PubMed] [Google Scholar]
58.Ho H.V.T., Sievenpiper J.L., Zurbau A., Mejia S.B., Jovanovski E., Au-Yeung F., Jenkins A.L., Vuksan V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016;116:1369–1382. doi: 10.1017/S000711451600341X. [DOI] [PubMed] [Google Scholar]
59.Tong L.-T., Zhong K., Liu L., Zhou X., Qiu J., Zhou S. Effects of dietary hull-less barley β-glucan on the cholesterol metabolism of hypercholesterolemic hamsters. Food Chem. 2015;169:344–349. doi: 10.1016/j.foodchem.2014.07.157. [DOI] [PubMed] [Google Scholar]
60.Zhang H., Cao X., Yin M., Wang J. Soluble dietary fiber from Qing Ke (highland barley) brewers spent grain could alter the intestinal cholesterol efflux in Caco-2 cells. J. Funct. Foods. 2018;47:100–106. doi: 10.1016/j.jff.2018.05.046. [DOI] [Google Scholar]
61.Byun A.R., Chun H., Lee J., Lee S.W., Lee H.S., Shim K.W. Effects of a dietary supplement with barley sprout extract on blood cholesterol metabolism. Evid.-Based Complement. Altern. Med. 2015;2015:473056. doi: 10.1155/2015/473056. [DOI] [PMC free article] [PubMed] [Google Scholar]
62.Xia X., Li G., Song J., Zheng J., Kan J. Hypocholesterolaemic effect of whole-grain highland hull-less barley in rats fed a high-fat diet. Br. J. Nutr. 2018;119:1102–1110. doi: 10.1017/S0007114518000831. [DOI] [PubMed] [Google Scholar]
63.Keenan J.M., Goulson M., Shamliyan T., Knutson N., Kolberg L., Curry L. The effects of concentrated barley β-glucan on blood lipids in a population of hypercholesterolaemic men and women. Br. J. Nutr. 2007;97:1162–1168. doi: 10.1017/S0007114507682968. [DOI] [PubMed] [Google Scholar]
64.Biörklund M., Van Rees A., Mensink R.P., Önning G. Changes in serum lipids and postprandial glucose and insulin concentrations after consumption of beverages with β-glucans from oats or barley: A randomised dose-controlled trial. Eur. J. Clin. Nutr. 2005;59:1272–1281. doi: 10.1038/sj.ejcn.1602240. [DOI] [PubMed] [Google Scholar]
65.Maki K.C., Davidson M.H., Ingram K.A., Veith P.E., Bell M., Gugger E. Lipid responses to consumption of a beta-glucan containing ready-to-eat cereal in children and adolescents with mild-to-moderate primary hypercholesterolemia. Nutr. Res. 2003;23:1527–1535. doi: 10.1016/S0271-5317(03)00178-7. [DOI] [Google Scholar]
66.Cicero A.F., Fogacci F., Veronesi M., Strocchi E., Grandi E., Rizzoli E., Poli A., Marangoni F., Borghi C. A randomized placebo-controlled clinical trial to evaluate the medium-term effects of oat fibers on human health: The beta-glucan effects on lipid profile, glycemia and intestinal health (BELT) study. Nutrients. 2020;12:686. doi: 10.3390/nu12030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
67.Wang Y., Harding S.V., Eck P., Thandapilly S.J., Gamel T.H., Abdel-Aal E.-S.M., Crow G.H., Tosh S.M., Jones P.J., Ames N.P. High-molecular-weight β-glucan decreases serum cholesterol differentially based on the CYP7A1 rs3808607 polymorphism in mildly hypercholesterolemic adults. J. Nutr. 2015;146:720–727. doi: 10.3945/jn.115.223206. [DOI] [PubMed] [Google Scholar]
68.Rondanelli M., Opizzi A., Monteferrario F., Klersy C., Cazzola R., Cestaro B. Beta-glucan-or rice bran-enriched foods: A comparative crossover clinical trial on lipidic pattern in mildly hypercholesterolemic men. Eur. J. Clin. Nutr. 2011;65:864–871. doi: 10.1038/ejcn.2011.48. [DOI] [PubMed] [Google Scholar]
69.Thomas S., Rezoagli E., Abidin I.Z., Major I., Murray P., Murphy E.J. β-Glucans from yeast—Immunomodulators from novel waste resources. Appl. Sci. 2022;12:5208. doi: 10.3390/app12105208. [DOI] [Google Scholar]
70.Carvalho A., Giovannini G., De Luca A., D’Angelo C., Casagrande A., Iannitti R.G., Ricci G., Cunha C., Romani L. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell. Mol. Immunol. 2012;9:276–286. doi: 10.1038/cmi.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
71.Auinger A., Riede L., Bothe G., Busch R., Gruenwald J. Yeast (1,3)-(1,6)-beta-glucan helps to maintain the body’s defence against pathogens: A double-blind, randomized, placebo-controlled, multicentric study in healthy subjects. Eur. J. Nutr. 2013;52:1913–1918. doi: 10.1007/s00394-013-0492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
72.Meng M., Guo M., Feng C., Wang R., Cheng D., Wang C. Water-soluble polysaccharides from Grifola Frondosa fruiting bodies protect against immunosuppression in cyclophosphamide-induced mice via JAK2/STAT3/SOCS signal transduction pathways. Food Funct. 2019;10:4998–5007. doi: 10.1039/C8FO02062K. [DOI] [PubMed] [Google Scholar]
73.Kopiasz Ł., Dziendzikowska K., Gajewska M., Oczkowski M., Majchrzak-Kuligowska K., Królikowski T., Gromadzka-Ostrowska J. Effects of dietary oat beta-glucans on colon apoptosis and autophagy through tlrs and dectin-1 signaling pathways—crohn’s disease model study. Nutrients. 2021;13:321. doi: 10.3390/nu13020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
74.Trivedi S., Patel K., Belgamwar V., Wadher K. Functional polysaccharide lentinan: Role in anti-cancer therapies and management of carcinomas. Pharmacol. Res.-Mod. Chin. Med. 2022;2:100045. doi: 10.1016/j.prmcm.2022.100045. [DOI] [Google Scholar]
75.Ying Y., Hao W. Immunomodulatory function and anti-tumor mechanism of natural polysaccharides: A review. Front. Immunol. 2023;14:1147641. doi: 10.3389/fimmu.2023.1147641. [DOI] [PMC free article] [PubMed] [Google Scholar]
76.Barsanti L., Passarelli V., Evangelista V., Frassanito A.M., Gualtieri P. Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Nat. Prod. Rep. 2011;28:457–466. doi: 10.1039/c0np00018c. [DOI] [PubMed] [Google Scholar]
77.Stier H., Ebbeskotte V., Gruenwald J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014;13:38. doi: 10.1186/1475-2891-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
78.de Graaff P., Govers C., Wichers H.J., Debets R. Consumption of β-glucans to spice up T cell treatment of tumors: A review. Expert Opin. Biol. Ther. 2018;18:1023–1040. doi: 10.1080/14712598.2018.1523392. [DOI] [PubMed] [Google Scholar]
79.Hong F., Yan J., Baran J.T., Allendorf D.J., Hansen R.D., Ostroff G.R., Xing P.X., Cheung N.-K.V., Ross G.D. Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
80.Małaczewska J., Wojcik R., Jung L., Siwicki A.K. Effect of Biolex β-HP on selected parameters of specific and non-specific humoral and cellular immunity in rats. Bull. Vet. Inst. Pulawy. 2010;54:75–80. [Google Scholar]
81.Qi C., Cai Y., Gunn L., Ding C., Li B., Kloecker G., Qian K., Vasilakos J., Saijo S., Iwakura Y., et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood J. Am. Soc. Hematol. 2011;117:6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
82.Liu M., Luo F., Ding C., Albeituni S., Hu X., Ma Y., Cai Y., McNally L., Sanders M.A., Jain D., et al. Dectin-1 activation by a natural product β-glucan converts immunosuppressive macrophages into an M1-like phenotype. J. Immunol. 2015;195:5055–5065. doi: 10.4049/jimmunol.1501158. [DOI] [PMC free article] [PubMed] [Google Scholar]
83.Hyder S., Dutta S.D. Mushroom-derived polysaccharides as antitumor and anticancer agent: A concise review. Biocatal. Agric. Biotechnol. 2021;35:102085. doi: 10.1016/j.bcab.2021.102085. [DOI] [Google Scholar]
84.Ali M.F., Driscoll C.B., Walters P.R., Limper A.H., Carmona E.M. β-glucan–activated human B lymphocytes participate in innate immune responses by releasing proinflammatory cytokines and stimulating neutrophil chemotaxis. J. Immunol. 2015;195:5318–5326. doi: 10.4049/jimmunol.1500559. [DOI] [PMC free article] [PubMed] [Google Scholar]
85.Wang D., Cui Q., Yang Y.J., Liu A., Zhang G., Yu J.C. Application of dendritic cells in tumor immunotherapy and progress in the mechanism of anti-tumor effect of astragalus polysaccharide (APS) modulating dendritic cells: A review. Biomed. Pharmacother. 2022;155:11354. doi: 10.1016/j.biopha.2022.113541. [DOI] [PubMed] [Google Scholar]
86.Chan S.-L., Yeung J.H. Effects of polysaccharide peptide (PSP) from Coriolus versicolor on the pharmacokinetics of cyclophosphamide in the rat and cytotoxicity in HepG2 cells. Food Chem. Toxicol. 2006;44:689–694. doi: 10.1016/j.fct.2005.10.001. [DOI] [PubMed] [Google Scholar]
87.Zhou X., Hao Q., Lu H. Mutant p53 in cancer therapy—The barrier or the path. J. Mol. Cell Biol. 2019;11:293–305. doi: 10.1093/jmcb/mjy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
88.Weitberg A.B. A phase I/II trial of beta-(1,3)/(1,6) D-glucan in the treatment of patients with advanced malignancies receiving chemotherapy. J. Exp. Clin. Cancer Res. 2008;27:40. doi: 10.1186/1756-9966-27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
89.Kodama N., Komuta K., Nanba H. Can maitake MD-fraction aid cancer patients? Altern. Med. Rev. 2002;7:236–239. [PubMed] [Google Scholar]
90.Parzonko A., Makarewicz-Wujec M., Jaszewska E., Harasym J., Kozłowska-Wojciechowska M. Pro-apoptotic properties of (1,3)(1,4)-β-D-glucan from Avena sativa on human melanoma HTB-140 cells in vitro. Int. J. Biol. Macromol. 2015;72:757–763. doi: 10.1016/j.ijbiomac.2014.09.033. [DOI] [PubMed] [Google Scholar]
91.Choromanska A., Kulbacka J., Rembialkowska N., Pilat J., Oledzki R., Harasym J., Saczko J. Anticancer properties of low molecular weight oat beta-glucan–An in vitro study. Int. J. Biol. Macromol. 2015;80:23–28. doi: 10.1016/j.ijbiomac.2015.05.035. [DOI] [PubMed] [Google Scholar]
92.Guicciardi M.E., Gores G.J. Life and death by death receptors. FASEB J. 2009;23:1625. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
93.Kim M.-J., Hong S.-Y., Kim S.-K., Cheong C., Park H.-J., Chun H.-K., Jang K.-H., Yoon B.-D., Kim C.-H., Kang S.A. β-Glucan enhanced apoptosis in human colon cancer cells SNU-C4. Nutr. Res. Pract. 2009;3:180–184. doi: 10.4162/nrp.2009.3.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
94.Lee S.H., Hwang H.S., Yun J.W. Antitumor activity of water extract of a mushroom, Inonotus obliquus, against HT-29 human colon cancer cells. Phytother. Res. 2009;23:1784–1789. doi: 10.1002/ptr.2836. [DOI] [PubMed] [Google Scholar]
95.Kobayashi H., Yoshida R., Kanada Y., Fukuda Y., Yagyu T., Inagaki K., Kondo T., Kurita N., Suzuki M., Kanayama N., et al. Suppressing effects of daily oral supplementation of beta-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. J. Cancer Res. Clin. Oncol. 2005;131:527–538. doi: 10.1007/s00432-005-0672-1. [DOI] [PubMed] [Google Scholar]
96.Youn M.-J., Kim J.-K., Park S.-Y., Kim Y., Park C., Kim E.S., Park K.-I., So H.S., Park R. Potential anticancer properties of the water extract of Inontus obliquus by induction of apoptosis in melanoma B16–F10 cells. J. Ethnopharmacol. 2009;121:221–228. doi: 10.1016/j.jep.2008.10.016. [DOI] [PubMed] [Google Scholar]
97.Zhao S., Ye G., Fu G., Cheng J.-X., Yang B.B., Peng C. Ganoderma lucidum exerts anti-tumor effects on ovarian cancer cells and enhances their sensitivity to cisplatin. Int. J. Oncol. 2011;38:1319–1327. doi: 10.3892/ijo.2011.965. [DOI] [PubMed] [Google Scholar]
98.Xu H., Zou S., Xu X. The β-glucan from Lentinus edodes suppresses cell proliferation and promotes apoptosis in estrogen receptor positive breast cancers. Oncotarget. 2017;8:86693. doi: 10.18632/oncotarget.21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
99.Queiroz E.A., Fortes Z.B., da Cunha M.A., Barbosa A.M., Khaper N., Dekker R.F. Antiproliferative and pro-apoptotic effects of three fungal exocellular β-glucans in MCF-7 breast cancer cells is mediated by oxidative stress, AMP-activated protein kinase (AMPK) and the Forkhead transcription factor, FOXO3a. Int. J. Biochem. Cell Biol. 2015;67:14–24. doi: 10.1016/j.biocel.2015.08.003. [DOI] [PubMed] [Google Scholar]
100.Xiao W., Su J., Gao X., Yang H., Weng R., Ni W., Gu Y. The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome. 2022;10:62. doi: 10.1186/s40168-022-01255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
101.Foysal J., Fotedar R., Siddik M.A.B., Tay A. Lactobacillus acidophilus and L. plantarum improve health status, modulate gut microbiota and innate immune response of marron (Cherax cainii) Sci. Rep. 2020;10:5916. doi: 10.1038/s41598-020-62655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
102.Sugiyama Y., Mori Y., Nara M., Kotani Y., Nagai E., Kawada H., Kitamura M., Hirano R., Shimokawa H., Nakagawa A., et al. Gut bacterial aromatic amine production: Aromatic amino acid decarboxylase and its effects on peripheral serotonin production. Gut Microbes. 2022;14:2128605. doi: 10.1080/19490976.2022.2128605. [DOI] [PMC free article] [PubMed] [Google Scholar]
103.Santos C.R., Costa P.A.C.R., Vieira P.S., Gonzalez S.E.T., Correa T.L.R., Lima E.A., Mandelli F., Pirolla R.A.S., Domingues M.N., Cabral L., et al. Structural insights into β-1,3-glucan cleavage by a glycoside hydrolase family. Nat. Chem. Biol. 2020;16:920–929. doi: 10.1038/s41589-020-0554-5. [DOI] [PubMed] [Google Scholar]
104.Abo Ghanima M.M., El-Aziz A.E.A., Noreldin A.E., Atta M.S., Mousa S.A., El-Far A.H. β-glucan administration improves growth performance and gut health in New Zealand White and APRI rabbits with different breed responses. PLoS ONE. 2020;15:e0234076. doi: 10.1371/journal.pone.0234076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
105.Rahmani J., Miri A., Černevičiūtė R., Thompson J., de Souza N.N., Sultana R., Varkaneh H.K., Mousavi S.M., Hekmatdoost A. Effects of cereal beta-glucan consumption on body weight, body mass index, waist circumference and total energy intake: A meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019;43:131–139. doi: 10.1016/j.ctim.2019.01.018. [DOI] [PubMed] [Google Scholar]
106.Mitsou E.K., Panopoulou N., Turunen K., Spiliotis V., Kyriacou A. Prebiotic potential of barley derived β-glucan at low intake levels: A randomised, double-blinded, placebo-controlled clinical study. Food Res. Int. 2010;43:1086–1092. doi: 10.1016/j.foodres.2010.01.020. [DOI] [Google Scholar]
107.Binou P., Yanni A.E., Stergiou A., Karavasilis K., Konstantopoulos P., Perrea D., Tentolouris N., Karathanos V.T. Enrichment of bread with beta-glucans or resistant starch induces similar glucose, insulin and appetite hormone responses in healthy adults. Eur. J. Nutr. 2021;60:455–464. doi: 10.1007/s00394-020-02265-6. [DOI] [PubMed] [Google Scholar]
108.Ahmad K., Singh N. Evaluation of nutritional quality of developed functional bread fortified with mushroom and dates. Clar.-Int. Multidiscip. J. 2016;5:23–28. doi: 10.5958/2277-937X.2016.00004.6. [DOI] [Google Scholar]
109.Sharafbafi N., Tosh S.M., Alexander M., Corredig M. Phase behaviour, rheological properties, and microstructure of oat β-glucan-milk mixtures. Food Hydrocoll. 2014;41:274–280. doi: 10.1016/j.foodhyd.2014.03.030. [DOI] [Google Scholar]
110.Rinaldi L., Rioux L.-E., Britten M., Turgeon S.L. In vitro bioaccessibility of peptides and amino acids from yogurt made with starch, pectin, or β-glucan. Int. Dairy J. 2015;46:39–45. doi: 10.1016/j.idairyj.2014.09.005. [DOI] [Google Scholar]
111.Kittisuban P., Ritthiruangdej P., Suphantharika M. Optimization of hydroxypropylmethylcellulose, yeast β-glucan, and whey protein levels based on physical properties of gluten-free rice bread using response surface methodology. LWT-Food Sci. Technol. 2014;57:738–748. doi: 10.1016/j.lwt.2014.02.045. [DOI] [Google Scholar]
112.Zielke C., Lu Y., Nilsson L. Aggregation and microstructure of cereal β-glucan and its association with other biomolecules. Colloids Surf. A Physicochem. Eng. Asp. 2019;560:402–409. doi: 10.1016/j.colsurfa.2018.10.042. [DOI] [Google Scholar]
113.Liu J., Li Q., Zhai H., Zhang Y., Zeng X., Tang Y., Tashi N., Pan Z. Effects of the addition of waxy and normal hull-less barley flours on the farinograph and pasting properties of composite flours and on the nutritional value, textural qualities, and in vitro digestibility of resultant breads. J. Food Sci. 2020;85:3141–3149. doi: 10.1111/1750-3841.15401. [DOI] [PubMed] [Google Scholar]
114.Kurek M.A., Moczkowska M., Pieczykolan E., Sobieralska M. Barley β-d-glucan–modified starch complex as potential encapsulation agent for fish oil. Int. J. Biol. Macromol. 2018;120:596–602. doi: 10.1016/j.ijbiomac.2018.08.131. [DOI] [PubMed] [Google Scholar]
115.Ahmad A., Khalid N. Dietary fibers in modern food production: A special perspective with β-glucans. In: Grumezescu A.M., Holban A.M., editors. Biopolymers for Food Design: Handbook of Food Bioengineering. Academic Press; Cambridge, MA, USA: 2018. pp. 125–156. [DOI] [Google Scholar]
116.Lyly M., Salmenkallio-Marttila M., Suortti T., Autio K., Poutanen K., Lähteenmäki L. The sensory characteristics and rheological properties of soups containing oat and barley β-glucan before and after freezing. LWT-Food Sci. Technol. 2004;37:749–761. doi: 10.1016/j.lwt.2004.02.009. [DOI] [Google Scholar]
117.Kulkarni A., Yokota T., Suzuki S., Etoh H. Subcritical water extraction of barley to produce a functional drink. Biosci. Biotechnol. Biochem. 2008;72:236–239. doi: 10.1271/bbb.70520. [DOI] [PubMed] [Google Scholar]
118.Temelli F. Extraction and functional properties of barley β-glucan as affected by temperature and pH. J. Food Sci. 1997;62:1194–1201. doi: 10.1111/j.1365-2621.1997.tb12242.x. [DOI] [Google Scholar]
119.Du B., Bian Z., Xu B. Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms: A review. Phytother. Res. 2014;28:159–166. doi: 10.1002/ptr.4963. [DOI] [PubMed] [Google Scholar]
120.Delatte S.J., Evans J., Hebra A., Adamson W., Othersen H., Tagge E.P. Effectiveness of beta-glucan collagen for treatment of partial-thickness burns in children. J. Pediatr. Surg. 2001;36:113–118. doi: 10.1053/jpsu.2001.20024. [DOI] [PubMed] [Google Scholar]
121.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
122.Vetvicka V., Teplyakova T.V., Shintyapina A.B., Korolenko T.A. Effects of medicinal fungi-derived β-glucan on tumor progression. J. Fungi. 2021;7:250. doi: 10.3390/jof7040250. [DOI] [PMC free article] [PubMed] [Google Scholar]
123.Garcia J., Rodrigues F., Saavedra M.J., Nunes F.M., Marques G. Bioactive polysaccharides from medicinal mushrooms: A review on their isolation, structural characteristics and antitumor activity. Food Biosci. 2022;49:101955. doi: 10.1016/j.fbio.2022.101955. [DOI] [Google Scholar]
124.Vetvicka V. Anti-infectious and Anti-tumor Activities of β-glucans. Anticancer Res. 2020;40:3139–3145. doi: 10.21873/anticanres.14295. [DOI] [PubMed] [Google Scholar]
125.Murphy E.J., Rezoagli E., Major I., Rowan N.J., Laffey J.G. β-glucan metabolic and immunomodulatory properties and potential for clinical application. J. Fungi. 2020;6:356. doi: 10.3390/jof6040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
126.Córdova-Martínez A., Caballero-García A., Roche E., Noriega D.C. β-Glucans could be adjuvants for SARS-CoV-2 virus vaccines (COVID-19) Int. J. Environ. Res. Public Health. 2021;18:12636. doi: 10.3390/ijerph182312636. [DOI] [PMC free article] [PubMed] [Google Scholar]
127.Therkelsen S.P., Hetland G., Lyberg T., Lygren I., Johnson E. Cytokine levels after consumption of a medicinal Agaricus blazei murill-based mushroom extract, AndoSan™, in patients with Crohn’s disease and ulcerative colitis in a randomized single-blinded placebo-controlled study. Scand. J. Immunol. 2016;84:323–331. doi: 10.1111/sji.12476. [DOI] [PubMed] [Google Scholar]
128.Joyce S.A., Kamil A., Fleige L., Gahan C.G.M. The cholesterol-lowering effect of oats and oat β-glucan: Modes of action and potential role of bile acids and the microbiome. Front. Nutr. 2019;6:171. doi: 10.3389/fnut.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
129.Levanič T., Cigić B., Germ M., Polišenská I., Vaculová K., Pravst I., Ačko D.K., Kreft I. Differences in ratio of carbon stable Isotopes among barley grain milling fractions with various concentrations of beta-glucans. Molecules. 2023;28:5738. doi: 10.3390/molecules28155738. [DOI] [PMC free article] [PubMed] [Google Scholar]
130. [(accessed on 15 October 2023)]. Available online: https://www.marketsandmarkets.com/
131.Diaz M., Sanchez Y., Bennett T., Sun C.R., Godoy C., Tamanoi F., Duran A., Perez P. The Schizosaccharomyces pombe cwg2+ gene codes for the beta subunit of a geranylgeranyltransferase type I required for beta-glucan synthesis. EMBO J. 1993;12:5245–5254. doi: 10.1002/j.1460-2075.1993.tb06220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
132.Roemer T., Bussey H. Yeast beta-glucan synthesis: KRE6 encodes a predicted type II membrane protein required for glucan synthesis in vivo and for glucan synthase activity in vitro. Proc. Natl. Acad. Sci. USA. 1991;88:11295–11299. doi: 10.1073/pnas.88.24.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
133.Levin D.E., Bartlett-Heubusch E. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J. Cell Biol. 1992;116:1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
134.Roemer T., GParavicini, Payton M.A., Bussey H. Characterization of the yeast (1→6)-beta-glucan biosynthetic components, Kre6p and Skn1p, and genetic interactions between the PKC1 pathway and extracellular matrix assembly. J. Cell Biol. 1994;127:567–579. doi: 10.1083/jcb.127.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
135.Watanabe D., Abe M., Ohya Y. Yeast Lrg1p acts as a specialized RhoGAP regulating 1, 3-β-glucan synthesis. Yeast. 2001;18:943–951. doi: 10.1002/yea.742. [DOI] [PubMed] [Google Scholar]
136.Maheshwari G., Sowrirajan S., Joseph B. Extraction and isolation of β-glucan from grain sources—A review. J. Food Sci. 2017;82:1535–1545. doi: 10.1111/1750-3841.13765. [DOI] [PubMed] [Google Scholar]
137.Wood P.J. Relationships between solution properties of cereal β-glucans and physiological effects—A review. Trends Food Sci. Technol. 2004;15:313–320. doi: 10.1016/j.tifs.2003.03.001. [DOI] [Google Scholar]
138.McCleary B.V., Codd R. Measurement of (1→3),(1→4)-β-D-glucan in barley and oats: A streamlined enzymic procedure. J. Sci. Food Agric. 1991;55:303–312. doi: 10.1002/jsfa.2740550215. [DOI] [Google Scholar]
139.Sourki A.H., Koocheki A., Elahi M. Ultrasound-assisted extraction of β-d-glucan from hull-less barley: Assessment of physicochemical and functional properties. Int. J. Biol. Macromol. 2017;95:462–475. doi: 10.1016/j.ijbiomac.2016.10.111. [DOI] [PubMed] [Google Scholar]
140.Harasym J., Olędzki R. Comparison of conventional and microwave assisted heating on carbohydrate content, antioxidant capacity and postprandial glycemic response in oat meals. Nutrients. 2018;10:207. doi: 10.3390/nu10020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
141.Chen J., Chen L., Lin S., Liu C., Cheung P.C.K. Preparation and structural characterization of a partially depolymerized beta-glucan obtained from Poria cocos sclerotium by ultrasonic treatment. Food Hydrocoll. 2015;46:1–9. doi: 10.1016/j.foodhyd.2014.12.005. [DOI] [Google Scholar]
142.Yang W., Huang G. Extraction methods and activities of natural glucans. Trends Food Sci. Technol. 2021;112:50–57. doi: 10.1016/j.tifs.2021.03.025. [DOI] [Google Scholar]
_1728976869676.webp)








