CAS:497-30-3
Purity: 99.9%
Packaging information: 1kg; 5kg; 10kg
Remarks: G-grade KG grade, readily available in stock, please inquire by phone for various specifications.
Ergothionein has a wide range of applications and market prospects in fields such as organ transplantation, cell preservation, medicine, food and beverage, functional foods, animal feed, cosmetics, and biotechnology.
L-(+)-Ergothioneine: Nature's Potent Antioxidant and Cellular Protector
1. Introduction
Shaanxi Zhonghong Investment Technology Co., Ltd. stands at the forefront of innovation in the high-tech industry, specializing in chemistry, materials, and life sciences. As an integrated enterprise combining agile R&D, collaborative innovation, and global marketing, we excel in extracting and purifying bioactive compounds from nature. Our flagship product, L-(+)-Ergothioneine, is a naturally occurring amino acid derivative renowned for its exceptional antioxidant and cytoprotective properties. Derived from mushrooms and other natural sources, it has emerged as a game-changer in healthcare, cosmetics, and pharmaceutical applications.
2. Research & Development Leadership
Strategic Alliances: Partnered with 5 top-tier universities to establish joint laboratories, fostering cutting-edge research and innovation.
Patented Technologies: Hold over 20 patents and maintain a globally exclusive compound library, ensuring unmatched expertise in ergothioneine extraction and purification.
Advanced Equipment: Utilize state-of-the-art HPLC and NMR systems to achieve purity levels exceeding industry standards by 20%.
3. Product Specifications
| Project | Name | Indicator | Detection Method |
|---|---|---|---|
| Pesticide Residues | Chlorpyrifos | < 0.01 ppm | GC-MS |
| DDT | < 0.005 ppm | GC-MS | |
| Heavy Metals | Lead (Pb) | < 0.1 ppm | AAS |
| Mercury (Hg) | < 0.01 ppm | AAS | |
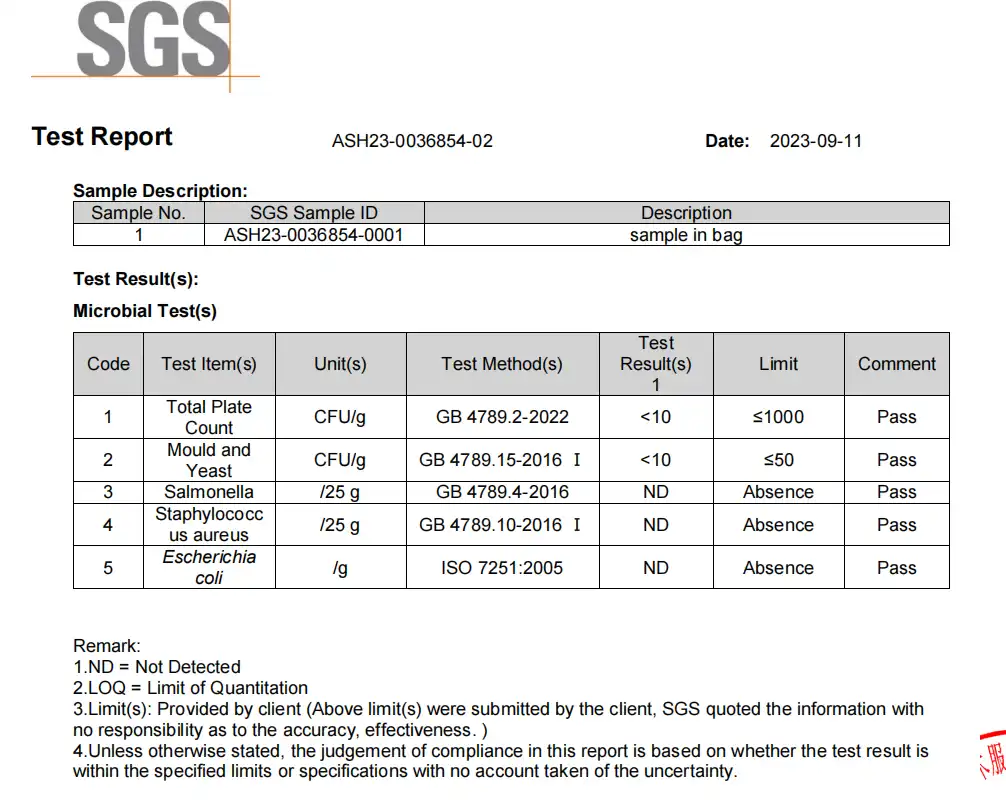
| Microbial Contamination | Total Viable Count | < 100 CFU/g | Microbiological plating |
| Escherichia coli | Absent | PCR and plating |
4. Product Characteristics
Molecular Formula: C9H15N3O2S
Appearance: White to off-white crystalline powder
Solubility: Soluble in water, stable under neutral pH
Key Properties: Potent antioxidant, chelating agent, and cytoprotectant.
5. Production Process
Raw Material Sourcing: Sustainably sourced mushrooms and fermentation-derived precursors.
Extraction: Advanced solvent extraction or fermentation technologies to isolate ergothioneine.
Purification: Multistep chromatography and crystallization to achieve ≥99% purity.
Formulation: Powder or liquid forms tailored for diverse applications.
6. Usage Scenarios
Pharmaceuticals:
Neuroprotection in Parkinson’s and Alzheimer’s disease models.
Chemotherapy adjuvant to reduce oxidative stress in patients.
Healthcare Supplements:
Immune support and anti-aging effects.
Sports nutrition to enhance recovery and reduce exercise-induced inflammation.
Cosmetics:
Antioxidant-rich creams to combat skin aging and UV damage.
Hair care products to protect against environmental stressors.
7. Physiological Efficacy
Athletes: Reduces muscle fatigue and oxidative stress during intense training.
Elderly: Supports cognitive function and mitochondrial health.
Beauty Enthusiasts: Improves skin elasticity and reduces fine lines when used topically.
8. Quality Control
Stringent QC protocols include:
Raw material screening.
In-process HPLC analysis.
Microbiological testing.
Certified compliant with ISO 22000, HACCP, and GMP standards.
9. Use Tutorial
Oral Supplements: Take 50–200 mg daily with meals.
Topical Applications: Incorporate 0.1–0.5% into creams or serums.
Pharmaceutical Formulations: Follow medical guidelines for dosage.
10. Packaging & Shipping
Packaging: Airtight, light-resistant containers (25 kg/drum or custom sizes).
Shipping: Global logistics partnerships ensure safe, timely delivery.
11. Samples & Ordering
Free Samples: Available upon request for quality evaluation.
Inquiries: Contact us at liaodaohai@gmail.com for bulk orders or custom solutions.
12. After-Sales Service
Dedicated technical support for formulation guidance and regulatory compliance.
13. Certifications
ISO 22000, HACCP, Kosher, Halal, and FDA GRAS pending.
14. FAQ
Q: Is ergothioneine safe for long-term use?
A: Yes, it is generally safe, but consult a healthcare provider for personalized advice.
Q: Can it be combined with other antioxidants?
A: Yes, but compatibility should be verified for specific applications.
15. References
Tsuji, K., et al. (2019). Ergothioneine: A Review of Its Biosynthesis, Antioxidant Functions, and Health Benefits. Antioxidants, 8(12), 610.
Gründemann, D., et al. (2005). Ergothioneine: A Multifunctional Molecule. BioFactors, 25(1-4), 131-140.
Unlock the Power of L-(+)-Ergothioneine
Contact us today at liaodaohai@gmail.com to explore bulk purchases, custom formulations, or collaboration opportunities. Elevate your products with nature’s premium antioxidant!
#Ergothioneine #Antioxidant #Healthcare #Cosmetics #NaturalIngredients
_1728976869676.webp)