Enhancing physical characteristics and antibacterial efficacy of chitosan through investigation of microwave-assisted chemically formulated chitosan-coated ZnO and chitosan/ZnO physical composite
Article Open access23 April 2024
2021-11-12
Chitosan is a highly valuable biopolymer, typically obtained as a byproduct from the shells of crustaceans. In this study, chitosan was derived from an herbal source for the first time, specifically from Ganoderma lucidum spore powder (GLSP). Both standard and emerging methods were employed for chitosan preparation: thermochemical deacetylation (TCD) and ultrasound-assisted deacetylation (USAD). The resulting chitosan was characterized through elemental analysis, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), and thermogravimetric measurements. The chitosan obtained through both methods exhibited comparable degrees of deacetylation (DD), viscosity ([η]), and molecular weight (Mv¯) to those of commercially available products. Both TCD and USAD methods produced chitosan that demonstrated excellent biocompatibility. Notably, the chitosan prepared by the USAD method showed significantly improved viability of fibroblast (document number 1) cells and enhanced antibacterial activity against both Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). These findings highlight the potential of the newly developed herbal chitosan as a significant advancement in natural biomaterials, suggesting a possible shift away from traditional sea-based sources.

Article Open access23 April 2024
Article Open access10 July 2023

Article Open access18 February 2021
Chitin is a white, linear polysaccharide that is insoluble in most common solvents. It is the main component of the exoskeletons of various insects and shellfish, such as crabs and shrimp. Due to its well-documented biodegradability and low toxicity, refined chitin is extensively used in biomaterial applications. Chitin is composed of N-acetyl-D-glucosamine units linked by β-(1–4) glycosidic bonds and is categorized into three polymeric forms: α, β, and γ. The most commonly used chitin comes from seafood shells, which may contain allergenic contaminants and a higher mineral content compared to fungal sources. This often necessitates complex purification methods to minimize health risks for consumers. Additionally, extracting high-quality chitin from discarded or spoiled seafood shells poses challenges that impact production time and safety.
Chitosan, a derivative of chitin, is produced through deacetylation, converting the GlcNAc unit in chitin to GlcN. Like chitin, chitosan is a biopolymer that has attracted significant research interest, surpassing even that of cellulose due to its superior solubility in water. Chitosan is biocompatible, biodegradable, and low in toxicity, and it effectively absorbs heavy metal ions, making it valuable in various applications, including food, pharmaceuticals, and biomedical engineering.
In response to the increasing demand for chitosan—driven by emerging bio-applications and a preference for natural materials—acetylation methods and processing techniques for chitin have diversified. Traditionally, thermochemical deacetylation (TCD) employs high concentrations of sodium hydroxide, elevated temperatures (around 120°C), and long reaction times (approximately 4 hours). To improve yield and quality, several alternative methods for converting chitin to chitosan have been developed. These include microwave-assisted deacetylation, ultrasound-assisted deacetylation (USAD), and supercritical fluid extraction. USAD has emerged as a significant advancement, converting β-chitin to chitosan with 83–94% deacetylation at lower temperatures (50–80°C) and much shorter reaction times (10–60 minutes), reducing the risk of severe depolymerization. Chitosan is commonly sourced from marine organisms, and pursuing alternative sources and improved processing methods will facilitate timely advancements.
Ganoderma lucidum, commonly known as the "Lingzhi" mushroom, belongs to a large group of fungi called polypores and has been used in traditional medicine for centuries. Recent studies have highlighted potential health benefits of Ganoderma lucidum spore powder (GLSP), which include improved immunoregulation, anti-inflammatory effects, anticancer properties, radical scavenging ability, and diabetes prevention. The powder features a chitin bilayer structure with an overall particulate size of approximately 6–12 μm. GLSP comprises a range of bioactive components, including polysaccharides, triterpenoids, nucleotides, sterols, steroids, and ganoderic acids, making it a promising nutritional source.
To date, several raw chitin products have primarily been developed from seafood byproducts, such as crab, shrimp, and lobster shells, with limited emphasis on harnessing the nutritional resources following human consumption. Given the growing demand and application of these materials, coupled with their nutritional composition, it is essential to explore opportunities to make these bioactive components more accessible.
Figure 1

Digital image of Ganoderma lucid powdeFull-sizeize image
In this study, chitin is derived from the herbal source (GLSP) for the first time. Chitosan synthesis is demonstrated using both TCD and USAD processes. A comparison between process conversion efficiency is shown and a detailed parametric study on USAD preparation from GLSP is detailed. The impact of USAD and TCD on herbal-sourced chitosan is reflected through comparison with commercially obtained chitosan. The extracted chitosan was characterized by employing FT-IR, TGA, XRD, and SEM. The bioassay indicates an exciting development for sourcing an important biopolymer which is crucial and timely for several biomass and bio-based industries.
GLSP is an excellent source of chitin, highlighting its potential for use and benefits in the biomedical and therapeutic fields. However, the process of converting raw herbal powder into chitosan has yet to be fully explored. This study utilized conventional TCD (Thermal Convention Deacetylation) and emerging USAD (Ultrasound-Assisted Deacetylation) methods to extract chitosan from fungal spores.
After deacetylation, the morphology of the chitosan changed depending on the method used (see Fig. 2). While numerous small pores were observed on the surface of GLSP, its overall particulate structure remained oval-shaped and rigid (Fig. 2a). This suggests that the polysaccharide extraction process does not affect the chitin structure. However, distinct morphological variations emerged after deacetylation, influenced by the method applied. The chitosan obtained through TCD (referred to as C-T hereafter) lost its well-defined oval shape and had a relatively smooth surface compared to the chitosan obtained via USAD (referred to as C-U hereafter). C-T was produced using a solvent and heating-based reaction under static conditions, whereas C-U involved vigorous ultrasound cavitation.
Consequently, the USAD method expands the potential applications of chitosan derived from herbal sources, as the irregular surface of C-U increases the contact area with other materials, offering additional benefits.
Figure 2
The effect of deacetylation and SEM images of GLSP, C-T, and C-U (a) GLSP SEM (b) SEM of C-T (c) SEM of C-U, and the effect of deacetylation time on DD, [η] and Mv¯ using TCD (d) and USAD (e). C-U sample was generated using USCD setup as: 90 W, 15 min, 20 w/v% NaOH, 1:15 (g: mL).Full-sizee image
GLSP is an excellent source of chitin, highlighting its potential for use and benefits in the biomedical and therapeutic fields. However, the process of converting raw herbal powder into chitosan has yet to be fully explored. This study utilized conventional TCD (Thermal Convention Deacetylation) and emerging USAD (Ultrasound-Assisted Deacetylation) methods to extract chitosan from fungal spores.
After deacetylation, the morphology of the chitosan changed depending on the method used (see Fig. 2). While numerous small pores were observed on the surface of GLSP, its overall particulate structure remained oval-shaped and rigid (Fig. 2a). This suggests that the polysaccharide extraction process does not affect the chitin structure. However, distinct morphological variations emerged after deacetylation, influenced by the method applied. The chitosan obtained through TCD (referred to as C-T hereafter) lost its well-defined oval shape and had a relatively smooth surface compared to the chitosan obtained via USAD (referred to as C-U hereafter). C-T was produced using a solvent and heating-based reaction under static conditions, whereas C-U involved vigorous ultrasound cavitation.
Consequently, the USAD method expands the potential applications of chitosan derived from herbal sources, as the irregular surface of C-U increases the contact area with other materials, offering additional benefits.
Figure 3
FTIR spectra of C-C, C-T and C-U (a); XRD analysis of GLSP, C-T and C-U (b).
Full-size image
As shown in Fig. 3b, the GLSP diffractogram exhibits a prominent peak at 2θ = 20.2°, corresponding to the (020, 110) plane. In comparison to the XRD data for C-T and C-U, the peak intensity at 2θ = 20.2° is significantly lower, indicating a reduction in crystallinity following deacetylation. Additionally, among the processed GLSP groups, the peak intensity (2θ = 20.2°) for C-T is lower than that for C-U, suggesting that temperature-controlled drying (TCD) reduces crystallinity more significantly than ultrasonic-assisted drying (USAD). Both chitosan materials display four distinct peaks at 2θ values of 27.5°, 31.86°, 45.64°, and 56.64° for C-T, and 27.5°, 31.86°, 45.58°, and 56.54° for C-U. The conversion process leads to peaks shifting toward higher 2θ values, and new intense peaks appear upon chitosan formation, indicating a change in the polymeric chain structure. According to the deacetylation degree (DD) values presented in Table 1, the maximum peak intensity at 2θ for GLSP is considerably higher compared to C-T and C-U, which aligns with previous research. Furthermore, the crystalline index (CrI) of chitosan, as noted in Table 1, is lower than that of GLSP, confirming the reduced crystallinity triggered by deacetylation.
Table 1 The DD, CrI and 2θ of GLSP, C-T and C-U.
Full size table
The TGA results for GLSP, C-T, and C-U are presented in Fig. 4. All materials exhibit a three-stage weight loss, consistent with previous findings. There is no significant difference in thermostability among the three samples. The onset degradation temperatures—defined as the temperature at which the sample weight begins to decrease—are 193°C for both GLSP and C-T and 210°C for C-U. For the GLSP sample, approximately 11.45% of weight loss occurs during the first stage (between 30°C and 226°C), attributed to surface-absorbed water and water bound to polymeric chains. As the temperature rises to 391°C, a weight loss of about 62.15% is noted for the GLSP sample, which is linked to the degradation of saccharides and deacetylated chitin units. In the third stage (from 391°C to 532°C), around 25.72% weight loss is observed due to polymer decomposition. The C-T and C-U samples show a similar pattern. However, the weight loss during the last two stages for both C-T and C-U primarily results from saccharide degradation. Notably, there are distinct differences in the final residue weight among the three samples, attributed to the content of chitin, proteins, and crude fats. These components are present in GLSP but are removed during the deacetylation process. Consequently, the residual weight after deacetylation of C-U is greater than that of C-T.
Figure 4
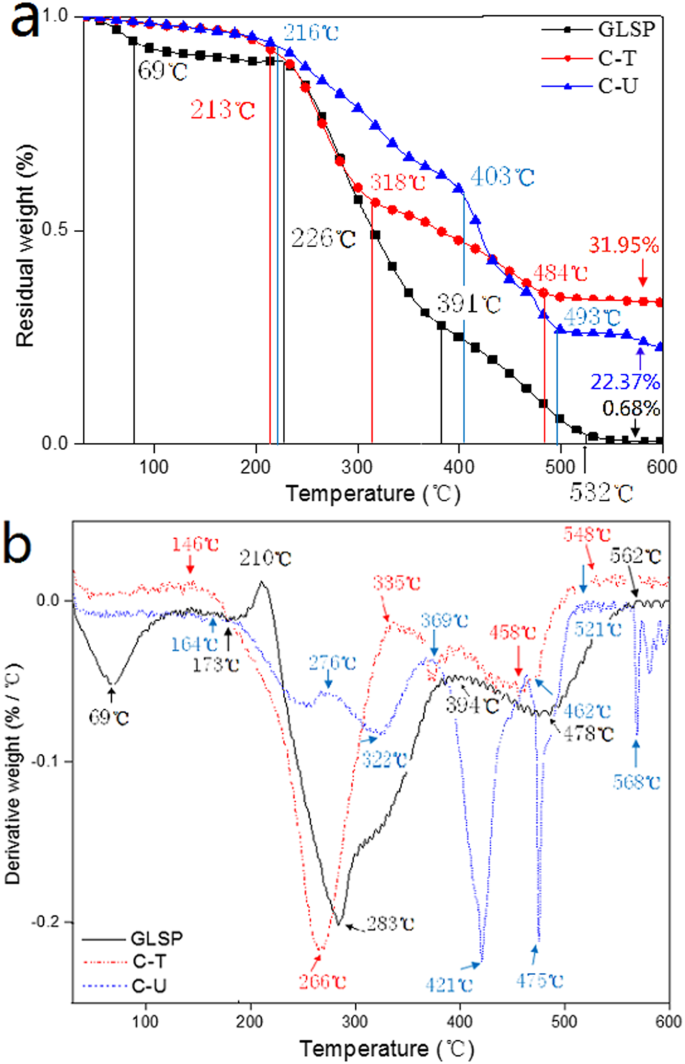
TGA analysis for GLSP, C-T and C-U. (a) Change in residual weight vs. temperature, (b) derivative weight vs. temperature.
Full-size image
Figure 4b shows temperature-related degradation on residue content for GLSP, C-T and C-U. Five peaks allude to the degradation velocity of chitin: 69, 210, 283, 394 and 478 °C, which indicate irregular degradation velocity upon temperature increase (from 30 to 600 °C). When the temperature is increased to 562 °C, the degradation velocity remains constant signaling the complete degradation of chitin. Derivative weight vs. temperature curves for C-T and C-U indicate degradation velocity for both samples is not constant. Complete degradation for C-T and C-U was noted at 548 and 521 °C, respectively. These results indicate the thermostability of chitosan is not altered during the deacetylation process, although degradation velocity is subject to variation.
The effect of GLSP, C-T, and C-U on the viability of mouse fibroblast cells was assessed using CCK-8 analysis. The addition of GLSP, C-T, C-U, and C-C to MEM improved cell viability, although the levels of improvement varied compared to the control group (see Fig. 5a). Specifically, C-U showed a greater improvement in cell viability compared to GLSP and C-T at a sample concentration of 1 mg/mL. The addition of GLSP, C-T, or C-U significantly enhanced cell viability, as determined by a T-test (p < 0.01).
The underlying mechanism by which GLSP and chitosan enhance cell viability involves the mediation of specific α-L-rhamnose-recognizing lectin sites that trigger the stimulation of oligosaccharides and polysaccharides. This mechanism also plays a role in collagen biosynthesis. Scanning Electron Microscopy (SEM) images (see Fig. 5b–d) demonstrated the adherence of GLSP and chitosan to the cell surface, further confirming the biocompatibility of chitosan. C-T adhered individually to the cell surface, exhibiting a nubby shape, likely due to the crystallization of C-T in the culture medium. In contrast, C-U adhered to the cell surface in a bunchy but dense form, which may be attributed to the rough surface characteristics that enhance cell adherence; the surface roughness of the C-U product was greater than that of the C-T sample. The combination of reduced size and rougher surface texture improved adherence to the cell surface.
While it has been previously reported that polysaccharides from abalone can accelerate proliferation in HepG2 cells, the specific impacts of GLSP, C-T, and C-U on cell viability warrant further investigation. This will help evaluate their potential as superior biomaterials compared to chitosan derived from conventional seafood byproducts. Merged and bright-field fluorescent micrographs (see Fig. 6a1–a4′) displayed intact cellular structures, including cell nuclei and cytoskeletons. Overall, the results indicate that the addition of GLSP and chitosan improves cell viability, highlighting their excellent biocompatibility and potential as a valuable biomass.
Figure 5
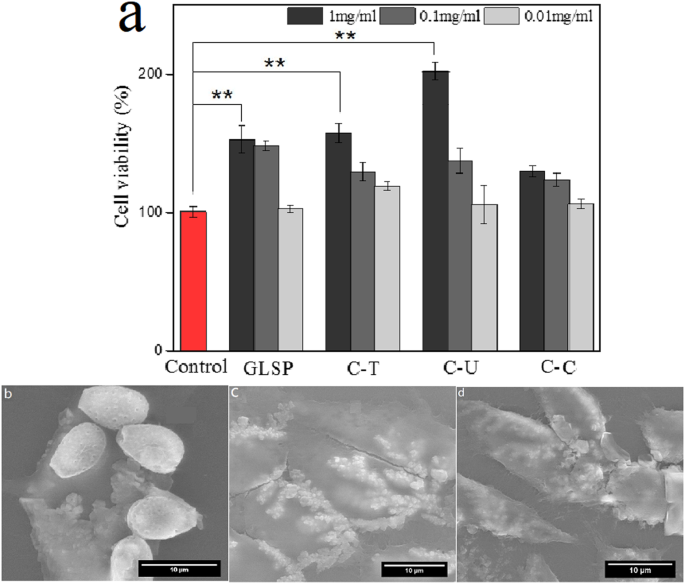
Cell viability evaluation. (a) Effects of GLSP/C-T/C-U/C-C with different concentration (1 mg/mL, 0.1 mg/mL, 0.01 mg/mL) on the L 929 cell viability using CCK-8 assay (b–d) SEM images of L929 cell morphology treated by GLSP/C-U/C-T (1 mg/mL). Each treatment condition was repeated using 8 wells (96-well plate).
Full size image
Figure 6
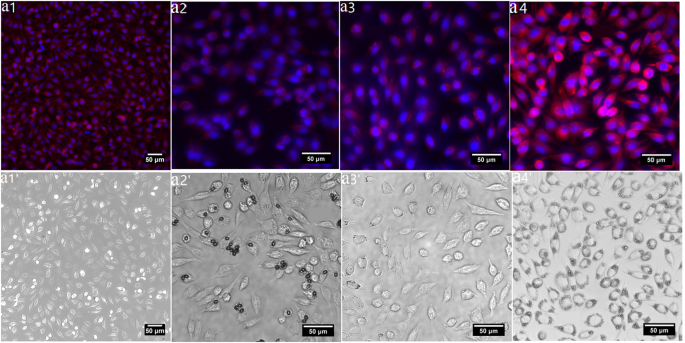
Biocompatibility assay using L929 cell. (a1–a4) Merged fluorescent images of L929 cell morphology treated by C-C/GLSP/C- U /C- T (1 mg/mL); (a1′–a4′) bright-field fluorescent image of (b1–b4) respectively.
Full size image
Antibacterial properties of GLSP, C-T, C-U and C-C were evaluated using the agar diffusion method. E. coli (Gram −) and S. aureus (Gram +) were selected as model bacteria. Antibacterial properties were quantified based on the inhibition zone surrounding the circular disc samples (Fig. 7). The inhibitory effect of C-U on both E. coli and S. aureus appears to be much greater than C-T, which may be due to shorter C-U molecular chains which favor penetration into bacterial cells (Table 2).
Figure 7
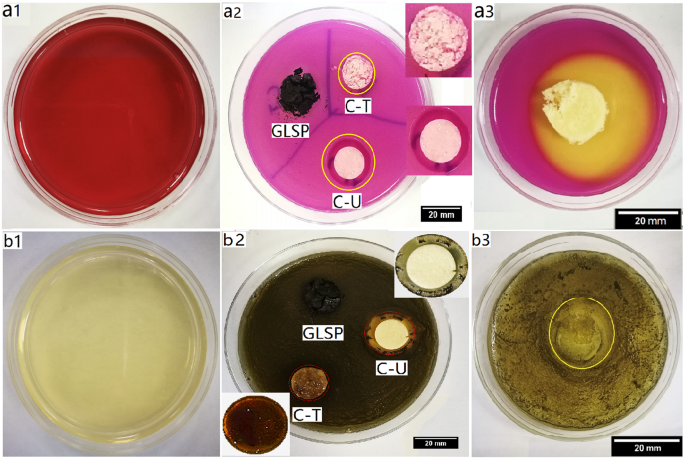
Effect of antibacterial activity assay. (a1) Violet red bile agar plate. (a2) Antibacterial effect of GLSP, C-T and C-U on E. coli post 24 h inoculation at 37 °C (b1) baird-parker agar plate. (b2) Antibacterial effect of GLSP, C-T and C-U on S. aureus post 24 h inoculation at 37 °C (a3 and b3) antibacterial effect of C-C on E. coli and S. aureus, respectively.
Full size image
Table 2 Inhibition zone obtained using agar plates method against E. coli and S. aureus.
Full size table
Changes in the integrity of the cytoplasmic membranes of E. coli and S. aureus following treatment with C-T or C-U were analyzed using a fluorescence microplate reader with propidium iodide (PI) and fluorescein diacetate (FDA) staining. The PI dye is effective for detecting non-viable cells as it binds to DNA and fluoresces red, while FDA can penetrate cell membranes, accumulates within living cells, and fluoresces green, making it ideal for assessing cell viability. Using both FDA and PI allows for a more accurate quantification of cell viability.
When E. coli was incubated with C-T or C-U at a concentration of 1 mg/mL, the proportion of living cells decreased from 100% in the control group to 26.38% and 15.34% for C-T and C-U, respectively. Conversely, the proportion of non-viable cells increased from 100% in the control group to 179.08% and 142.53%, respectively.
The results also indicated that the addition of C-T or C-U at a concentration of 1 mg/mL effectively inhibited the growth of S. aureus. Compared to the control group, the viable cell proportions decreased from 100% to 60.05% in the C-T group and to 23.05% in the C-U group. Additionally, the proportion of non-viable cells rose from 100% to 151.39% in the C-T group and to 110.81% in the C-U group.
Figure 8
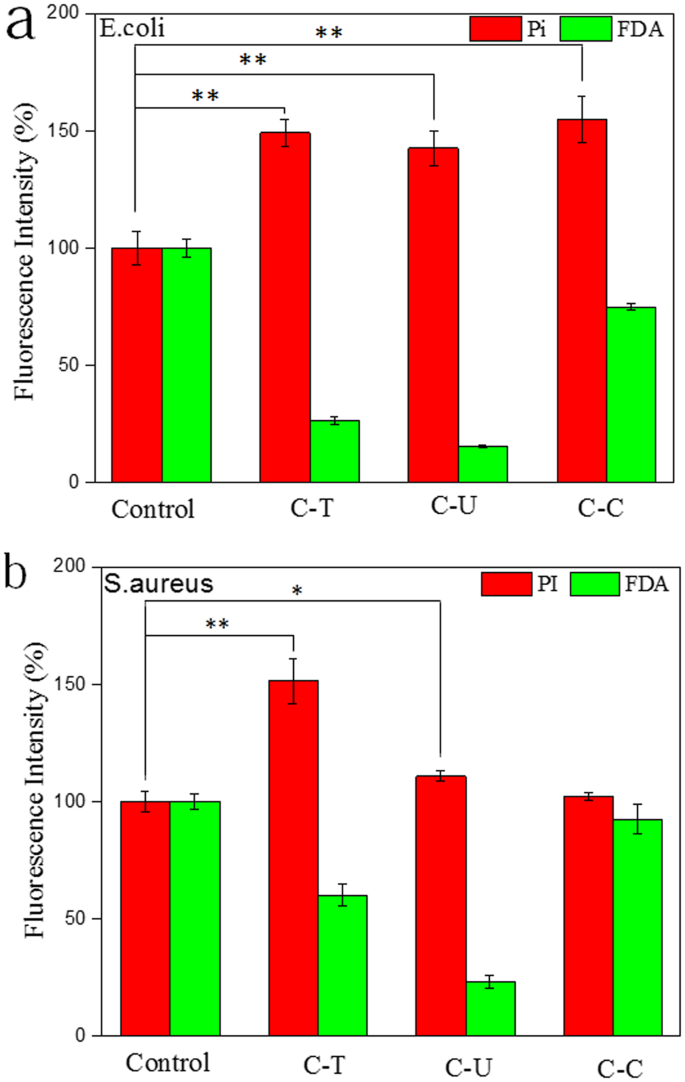
Fluorescence microplate reader analysis of membrane permeability. (a) Staining of E. coli using Pi and FDA (b) staining of S. aureus using Pi and FDA; 0.4 mL inoculum containing ~1.5 × 106 CFU/mL of either E. coli or S. aureus were incubated in each well (96-well plate) at 37 °C for 24 h. An identical concentration of chitosan (1 mg/mL) was added to each well. Eight wells were measured from each test group, **p < 0.01, *p < 0.05.
Full-size image
Chitosan has been reported to possess antibacterial and antifungal properties. Its inhibitory effects on gram-negative bacteria, such as E. coli, are attributed to its ability to bind to outer cell membranes, which increases the permeability of the bacterial cells. This increased permeability can result in the leakage of essential biological components. In contrast, the inhibitory effects on gram-positive bacteria, like S. aureus, are associated with the restriction of nutrient entry into the cells, ultimately leading to cell death. Fluorescence intensities of propidium iodide (PI) or fluorescein diacetate (FDA) suggest that the antibacterial effects of chitosan with a higher degree of deacetylation (C-T) were more significant than those of chitosan with a lower degree of deacetylation (C-U). This greater effectiveness is linked to a higher degree of deacetylation value obtained for C-T, as shown in Table 1. Various parameters known to influence antibacterial activity, such as molecular weight, structure, and the amount of positively charged content, also play a role.
The current work investigates the herbal-based source for chitosan production. Both TCD and USAD successfully induced N-deacetylation reaction and converted chitin into chitosan and products were compared with a commercial source. The obtained chitosan was characterized by elemental analysis, XRD, FT-IR and thermogravimetric measurements. Chitosan derived using both processes displayed excellent biocompatibility and improved fibroblast cell viability and antibacterial activity. Compared to control and TCD groups, chitosan prepared via USAD displayed better viability and antibacterial zones. These interesting findings indicate an exciting breakthrough for herbal-based chitosan for use in biomaterial science.
GLSP was provided by TianHe Agricultural Group located in Long Quan, Zhe Jiang, China. The relics of GLSP used in this study were prepared following polysaccharide extraction as previously described. Hydrochloric acid, acetic acid, sodium chloride (troche), and sodium hydroxide (troche) were sourced from Sinopharm Chemical Reagent Co., Ltd. in Shanghai, China. Modified Eagle's Medium (MEM) was obtained from Gibco (USA), and fetal bovine serum (FBS) was supplied by Sijiqin in Hangzhou, Zhejiang, China. Deionized water (DI) was produced using a Millipore Milli-Q Reference ultrapure water purifier (Millipore, Bedford, USA). Hydrogen peroxide (30% H2O2) was also purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals used were of analytical grade and did not require additional purification. For comparison purposes, standard (commercial) chitosan (C6nH11nNO4n) was acquired from Macklin Biochemical Co., Ltd. in Shanghai, China.
GLSP relics obtained post polysaccharide extraction were dried in a vacuum oven (−0.095 MPa, D2F-6020AF, Tianjin GongXing Laboratory Instrument Co., Ltd., Tianjin, China) at 65 °C for 3 days prior to further treatment. Dried GLSP was then bleached with 30% H2O2 at 70 °C for 2 h according to a previous method43. The pH of resulting suspension was adjusted to neutral using NaOH and universal indicator paper (pH 1–14, Shanghai SSS reagent Co., Ltd., Shanghai, China). Subsequently, the mixture was centrifuged at 7000 rpm for 10 min (Centrifuge 5810 R, Eppendorf, Germany), after which the GLSP sediment was transferred into a centrifuge tube and stored for further experimentation.
The GlcNAc unit in chitin is converted to GlcN to form chitosan, which consists of repeating units from both biopolymers. In the TCD process, a 20% (w/v) NaOH solution is prepared by dissolving NaOH flakes in deionized (DI) water. This alkaline solution is then added to GLSP sediment (0.5 g) at a solid-liquid ratio of 1:15 (g:mL) in a conical flask. Individual GLSP suspensions are heated to 90 °C in a thermostatic water bath (Model DK-8D, Jinghong Laboratory Instrument Co., Ltd., Shanghai, China) for predetermined time periods ranging from 1 to 3 hours.
After heating, hydrochloric acid (HCl) is added dropwise to adjust the pH, after which the neutral suspensions are allowed to stand overnight (for 14 hours). The suspensions are then centrifuged at 7000 rpm for 10 minutes, and the resulting sediments are collected and dried in a vacuum oven (0.95 MPa, Model D2F-6020AF, GongXing Laboratory Instrument Co., Ltd., Tianjin, China) at 65 °C for 3 days. This process yields the final product: chitosan.
For the ultrasound-assisted deacetylation (USAD) process, a 20% (w/v) NaOH solution is similarly prepared by dissolving NaOH flakes in DI water. The alkaline solution is added to GLSP sediment (0.5 g) at a solid-liquid ratio of 1:20 (g:mL) in a centrifuge tube. Several identical suspensions are then subjected to ultrasound irradiation for periods of 5 to 75 minutes at ambient temperature (25 °C) using an ultrasonic transducer (Branson Digital Sonifier 250, Danbury, Conn., USA). The temperature of the deionized water is monitored every 5 minutes using a mercurial thermometer (MC, 30 cm, 0 °C–200 °C), as the actual temperature of the irradiated sample varies with the duration of the irradiation (see Supplementary Fig. S1).
During the ultrasound irradiation process, the device is equipped with an ultrasonic probe (diameter = 1.3 cm, frequency = 20 kHz). The distance from the ultrasound probe to the centrifuge tube is maintained at 2 cm, and the total volume of DI water (2 L) in the glass container (20 × 15 × 15 cm) remains constant. Following the ultrasonic irradiation, HCl is added dropwise to the suspensions until neutralization is achieved. The suspensions are then allowed to stand overnight (for 14 hours). The resulting samples undergo centrifugation, and the sediments are collected and dried in a vacuum oven (−0.095 MPa, Model D2F-6020AF, Tianjin GongXing Laboratory Instrument Co., Ltd., Tianjin, China) at 65 °C for 3 days, yielding the final product: chitosan.
Field emission environmental scanning electron microscopy (SEM, Quanta FEG650, FEI, China) were used to study surface features of raw GLSP and resulting chitosan products. For SEM analysis, an accelerating voltage of 20 kV was used. Samples were fixed on metallic stubs using double-backed conductive tape. Prior to analysis, all samples were sputter-coated (108 Auto Cressington Sputter Coater, Ted Pella, INC.) with a thin layer of gold under vacuum for 60 s using a current intensity of 25 mA.
Cell viability was assessed using the CCK-8 (Cell Counting Kit-8 reagent, Dojindo Laboratories, Kumamoto, Japan). The cells were cultured in Modified Eagle’s Medium (MEM) (Gibco, Carlsbad, California, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) in 6 cm diameter cell culture dishes. The standard conditions for incubation were set at 37 °C in a humid atmosphere with 5% CO2 for 48 hours. A cell suspension with a density of 1.4 × 10^5 cells/mL was prepared after this period. Selected materials were disinfected using UV irradiation for 2 hours before being added to the cell culture medium.
Subsequently, 100 μL of the cell suspension was pipetted into a 96-well plate and incubated under standard conditions for 24 hours. Samples of GLSP, C-T, C-U, or C-C were then added at concentrations of 1, 0.1, and 0.01 mg/mL, and the plate was incubated for an additional 24 hours. After this incubation, 10 μL of CCK-8 solution was added, and the absorbance values from 8 wells (measured after 4 hours of incubation for each condition) were assessed using a microplate reader (Spectra Max 190, NanoDrop, USA) at 450 nm.
In addition, 1 mL of the original cell suspension was combined with 2 mL of MEM directly in a cell culture dish (Φ = 3 cm) and incubated under standard conditions for 24 hours. The media was then removed, and 3 mL of fresh medium was added along with 3 mg of GLSP, C-T, C-U, or C-C, followed by another 24-hour incubation. The effects of biopolymer growth on the cells were visualized using fluorescence microscopy (Olympus, BX61W1-FV1000, Tokyo, Japan). For staining, Alexa Fluor 546 phalloidin (Invitrogen, Carlsbad, California, USA) and 4′,6′-diamidino-2-phenylindole hydrochloride (DAPI, Invitrogen) were used, following a previously established procedure for cell treatment.
Stained cells underwent further analysis using Scanning Electron Microscopy (SEM) to evaluate cell and biopolymer interactions. For SEM analysis, the samples were coated with a layer of gold as described in the methods, and an accelerating voltage of 15 kV was employed.
Control groups consisted of cells incubated with MEM and CCK-8, while blank groups comprised mixtures of MEM and CCK-8 only. Cell viability in the control groups was defined as 100%, and cell viability in the treated groups was calculated using the following equation: (insert Eq. 8 here).
Cellviability(%ofuntreatedcells)=[(As−Ab)/(Ac−Ab)]×100%.
(8)
Here, As, Ac and Ab are the absorbance values for experimental, control and blank groups, respectively.
he antibacterial activity of GLSP, C-T, C-U, and C-C was assessed using a plate assay method. Inhibition zone assays were conducted to evaluate the antibacterial properties against two common pathogens: E. coli (plate number 1, catalog 8099, Nanjing Maojie Microbiology Technology Co. Ltd., Jiangsu, China) and S. aureus (CMCC (B) number 1, Shanghai Luwei Microbial Science & Technology Co., Ltd., China).
To prepare the assays, 300 mg of the selected biopolymers (e.g., GLSP, C-T, or C-U) were compressed into discs using a compressing machine (FW-4A, Tianjin TUOPU Instrument Co., Ltd., Tianjin, China). An inoculum containing approximately 1.5 × [plate number 2] /mL of either E. coli or S. aureus was added to violet red bile agar (Qingdao Hope Bio-Technology Co., Ltd., Shandong, China) plates and Baird-Parker agar base (Qingdao Hope Bio-Technology Co., Ltd., Shandong, China) plates, respectively. Egg-yolk tellurite emulsion was subsequently added to the Baird-Parker agar base culture system (Qingdao Hope Bio-Technology Co., Ltd., Shandong, China).
The compressed GLSP, C-T, or C-U discs were then placed on the inoculated agar plates (10 cm diameter) and incubated in a biochemical incubator (plate number 3, Shanghai Jinghong Laboratory Instrument Co., Ltd., Shanghai, China) at 37 °C for 24 hours. After incubation, the diameter of the inhibition zones was measured for all samples.
For fluorescence microplate reader analysis, an inoculum containing approximately 1.5 × [plate number 2] /mL of either E. coli or S. aureus was incubated in nutrient broth (Qingdao Hope Bio-Technology Co., Ltd., Shandong, China) and 7.5% sodium chloride broth (Qingdao Hope Bio-Technology Co., Ltd., Shandong, China), respectively. E. coli and S. aureus were stained with 25 μg/mL propidium iodide (PI) (Solarbio Life Science, Beijing, China) for 20 minutes and 25 μg/mL fluorescein diacetate (FDA) (Solarbio Life Science, Beijing, China) for 20 minutes at ambient temperature (25 °C) prior to fluorescence analysis. The fluorescence analysis was carried out using a fluorescent microplate reader (FlexStation II, NanoDrop, USA). Control groups consisted of bacteria that were incubated in broth only for 24 hours at 37 °C.
All experiments were performed in triplicate and data is given as mean ± standard deviation (n = 3). Statistical analysis was performed using SPSS software (SPSS Statistics v18, IBM, UK). All statistical data was plotted using Origin software (OrginLab, USA).
Kuroki, M. et al. Chitin-deacetylase activity induces appressorium differentiation in the rice blast fungus Magnaporthe oryzae. Scientific Reports 7, 9697 (2017).
Article ADS PubMed Central PubMed Google Scholar
Yan, N. & Chen, X. Don’t waste seafood waste: Turning cast-off shells into nitrogen-rich chemicals would benefit economies and the environment. Nature 524, 155–158 (2015).
Article ADS CAS PubMed Google Scholar
Zhu, K. K. et al. High-Strength Films Consisted of Oriented Chitosan Nanofibers for Guiding Cell Growth. Biomacromolecules 18, 3904–3912, https://doi.org/10.1021/acs.biomac.7b00936 (2017).
Article CAS PubMed Google Scholar
Wan, A. C. A. & Tai, B. C. U. Chitin— a promising biomaterial for tissue engineering and stem cell technologies. Biotechnology Advances 31, 1776–1785 (2013).
Article CAS PubMed Google Scholar
Álvarez, O. S. P. et al. Comparison of extraction methods of chitin from Ganoderma lucidum mushroom obtained in submerged culture. Biomed Research International 2014, 169071 (2014).
Article Google Scholar
Luo, J.-C. et al. A multi-step method for preparation of porcine small intestinal submucosa (SIS). Biomaterials 32, 706–713 (2011).
Article CAS PubMed Google Scholar
Yeung, R. & Morris, J. Food safety risk—Consumer perception and purchase behavior. Vol. 103 (2001).
Article Google Scholar
Khor, E. Chitin: Fulfilling a Biomaterials Promise: Second Edition. (The Netherlands Biomaterials 2001).
Chandumpai, A., Singhpibulporn, N., Faroongsarng, D. & Sornprasit, P. Preparation and physico-chemical characterization of chitin and chitosan from the pens of the squid species, Loligo lessoniana and Loligo formosana. Carbohydrate Polymers 58, 467–474 (2004).
Article CAS Google Scholar
Kurita, K. Chitin and chitosan: functional biopolymers from marine crustaceans. Marine Biotechnology 8, 203 (2006).
Article CAS PubMed Google Scholar
Roosen, J., Spooren, J. & Binnemans, K. Adsorption performance of functionalized chitosan–silica hybrid materials toward rare earths. Journal of Materials Chemistry A 2, 19415–19426 (2014).
Article CAS Google Scholar
Liu, M., Zhong, X. & Yang, Z. Chitosan functionalized nanocochleates for enhanced oral absorption of cyclosporine A. Scientific Reports 7, 41322 (2017).
Article ADS CAS PubMed Central PubMed Google Scholar
Ragetly, G. R. et al. Effect of chitosan scaffold microstructure on mesenchymal stem cell chondrogenesis. Acta Biomaterialia 6, 1430–1436 (2010).
Article CAS PubMed Google Scholar
Pariser, E. R. & Lombardi, D. P. Chitin sourcebook: a guide to the research literature. Wiley, New York (1989).
Noishiki, Y. et al. Alkali-Induced Conversion of β-Chitin to α-Chitin. Biomacromolecules 4, 896–899 (2003).
Article CAS PubMed Google Scholar
Sayari, N. et al. Chitin and chitosan from the Norway lobster by-products: Antimicrobial and anti-proliferative activities. International Journal of Biological Macromolecules 87, 163–171, https://doi.org/10.1016/j.ijbiomac.2016.02.057 (2016).
Article CAS PubMed Google Scholar
Dahmoune, F., Nayak, B., Moussi, K., Remini, H. & Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chemistry 166, 585–595 (2015).
Article CAS PubMed Google Scholar
Melo, M. M. R. D., Silvestre, A. J. D. & Silva, C. M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. Journal of Supercritical Fluids the 92, 115–176 (2014).
Article Google Scholar
Birolli, W. G., de Moura Delezuk, J. A. & Campana-Filho, S. P. Ultrasound-assisted conversion of alpha-chitin into chitosan. Applied Acoustics 103, 239–242 (2016).
Article Google Scholar
Khor, E. & Lim, L. Y. Implantable applications of chitin and chitosan. Biomaterials 24, 2339–2349 (2003).
Article CAS PubMed Google Scholar
Paterson, R. R. M. Ganoderma–a therapeutic fungal biofactory. Phytochemistry 67, 1985–2001 (2006).
Article CAS PubMed Google Scholar
Sanodiya, B. S., Thakur, G. S., Baghel, R. K., Prasad, G. B. & Bisen, P. S. Ganoderma lucidum: a potent pharmacological macrofungus. Current Pharmaceutical Biotechnology 10, 717–742 (2009).
Article CAS PubMed Google Scholar
Mohammed, M. H., Williams, P. A. & Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocolloids 31, 166–171 (2013).
Article CAS Google Scholar
Yue, W., He, R., Yao, P. & Wei, Y. Ultraviolet radiation-induced accelerated degradation of chitosan by ozone treatment. Carbohydrate Polymers 77, 639–642 (2009).
Article CAS Google Scholar
Kucukgulmez, A. et al. Physicochemical characterization of chitosan extracted from Metapenaeus stebbingi shells. Food Chemistry 126, 1144–1148 (2011).
Article CAS Google Scholar
Rinaudo, M. Chitin and chitosan: Properties and applications. Cheminform 38, 603–632 (2006).
Google Scholar
Lavall, R. L., Assis, O. B. & Campana-Filho, S. P. Beta-chitin from the pens of Loligo sp.: extraction and characterization. Bioresource Technology 98, 2465–2472 (2007).
Article CAS PubMed Google Scholar
Al Sagheer, F., Al-Sughayer, M., Muslim, S. & Elsabee, M. Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydrate Polymers 77, 410–419 (2009).
Article CAS Google Scholar
Prashanth, K. V. H., Kittur, F. S. & Tharanathan, R. N. Solid state structure of chitosan prepared under different N-deacetylating conditions. Carbohydrate Polymers 50, 27–33 (2002).
Article Google Scholar
Zhang, Y., Xue, C., Xue, Y., Gao, R. & Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydrate research 340, 1914–1917 (2005).
Article CAS PubMed Google Scholar
Delezuk, J. A. D. M., Cardoso, M. B., Domard, A. & Campana-Filho, S. P. Ultrasound-assisted deacetylation of beta-chitin: influence of processing parameters. Polymer International 60, 903–909 (2011).
Article CAS Google Scholar
Ospina, N. M. et al. Isolation of chitosan from Ganoderma lucidum mushroom for biomedical applications. J Mater Sci Mater Med 26, 135 (2015).
Article Google Scholar
Kaya, M. et al. First chitin extraction from Plumatella repens (Bryozoa) with comparison to chitins of insect and fungal origin. International Journal of Biological Macromolecules 79, 126 (2015).
Article CAS PubMed Google Scholar
Andrès, E. et al. Pharmacological properties of rhamnose-rich polysaccharides, potential interest in age-dependent alterations of connectives tissues. Pathologie-biologie 54, 420–425 (2006).
Article PubMed Google Scholar
Wang, Y. M. et al. Effects of polysaccharides from abalone (Haliotis discus hannai Ino) on HepG2 cell proliferation. International Journal of Biological Macromolecules 66, 354–361 (2014).
Article ADS CAS PubMed Google Scholar
C, P. V. et al. Cytometric approach for a rapid evaluation of susceptibility of Candida strains to antifungals. Clinical Microbiology & Infection 7, 609–618 (2001).
Article Google Scholar
Rhim, J. W., Hong, S. I., Park, H. M. & Ng, P. K. W. Preparation and Characterization of Chitosan-Based Nanocomposite Films with Antimicrobial Activity. Journal of Agricultural & Food Chemistry 54, 5814–5822 (2006).
Article CAS Google Scholar
Pan, C., Rezaei, H. & Soor, A. Chitosan Disrupts Membrane Permeability of Lactic Acid Bacteria. Journal of Experimental Microbiology and Immunology 15, 7–14 (2011).
Google Scholar
Jeon, Y. J. & Kim, S. K. Production of chitooligosaccharides using an ultrafiltration membrane reactor and their antibacterial activity. Carbohydrate Polymers 41, 133–141 (2000).
Article CAS Google Scholar
Takahashi, T., Imai, M., Suzuki, I. & Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochemical Engineering Journal 40, 485–491 (2008).
Article CAS Google Scholar
Yang, T. C., Li, C. F. & Chou, C. C. Cell age, suspending medium and metal ion influence the susceptibility of Escherichia coli O157:H7 to water-soluble maltose chitosan derivative. International Journal of Food Microbiology 113, 258–262 (2007).
Article CAS PubMed Google Scholar
Yao, Z. C. et al. Ganoderma lucidum polysaccharide loaded sodium alginate micro-particles prepared via electrospraying in controlled deposition environments. International Journal of Pharmaceutics 524 (2017).
Article CAS PubMed Google Scholar
Chen, C. C. et al. Non-shellfish chitosan from the fruiting body residue of Ganoderma tsugae for long-lasting antibacterial guided-tissue regeneration barriers. Journal of Dental Sciences 2, 19–29 (2007).
Google Scholar
Brugnerotto, J. et al. An infrared investigation in relation with chitin and chitosan characterization. Polymer 42, 3569–3580 (2001).
Article CAS Google Scholar
Lin, S. G. & Zhao, X. L. Polymer Chemistry. (Beijing: science Press, 1998).
Kasaai, M. R., Arul, J. & Charlet, G. Intrinsic viscosity–molecular weight relationship for chitosan. Journal of Polymer Science Part B Polymer Physics 38, 2591–2598 (2015).
Article ADS Google Scholar
Wei, W., Bo, S. Q., Li, S. Q. & Wen, Q. Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. International Journal of Biological Macromolecules 13, 281–285 (1991).
Article Google Scholar
Yao, Z. C., Chang, M. W., Ahmad, Z. & Li, J. S. Encapsulation of rose hip seed oil into fibrous zein films for ambient and on demand food preservation via coaxial electrospinning. Journal of Food Engineering 191, 115–123 (2016).
Article CAS Google Scholar
Wang, B., Zhou, W., Chang, M. W., Ahmad, Z. & Li, J. S. Impact of substrate geometry on electrospun fiber deposition and alignment. Journal of Applied Polymer Science 134 (2017).
Download references
This research was financially supported by the National Nature Science Foundation of China (No. 81771960), the Fundamental Research Funds for the Central Universities (2017QNA5017) and Key Technologies R&D Program of Zhejiang Province (2015C02035).
Key Laboratory for Biomedical Engineering of Education Ministry of China, Zhejiang University, Hangzhou, 310027, PR China
Li-Fang Zhu, Zhi-Cheng Yao & Ming-Wei Chang
Zhejiang Provincial Key Laboratory of Cardio-Cerebral Vascular Detection Technology and Medicinal Effectiveness Appraisal, Zhejiang University, Hangzhou, 310027, PR China
Li-Fang Zhu, Zhi-Cheng Yao, Jing-Song Li & Ming-Wei Chang
Leicester School of Pharmacy, De Montfort University, The Gateway, Leicester, LE1 9BH, UK
Zeeshan Ahmad
Li-Fang Zhu performed the experimental work. Zhi-Cheng Yao, Zeeshan Ahmad and Jing-Song Li helped with experiments, and analysed the data. Ming-Wei Chang supervised and designed the research. All authors revised the manuscript and reviewed the manuscript.
Correspondence to Ming-Wei Chang.
The authors declare no competing interests.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
YOU MAY LIKE